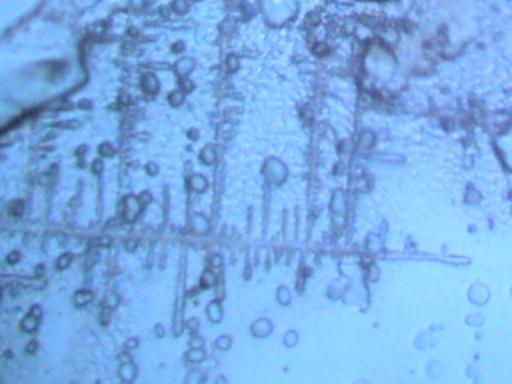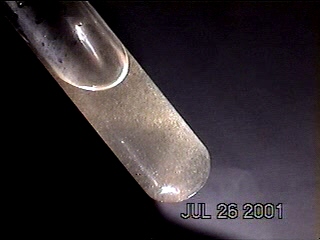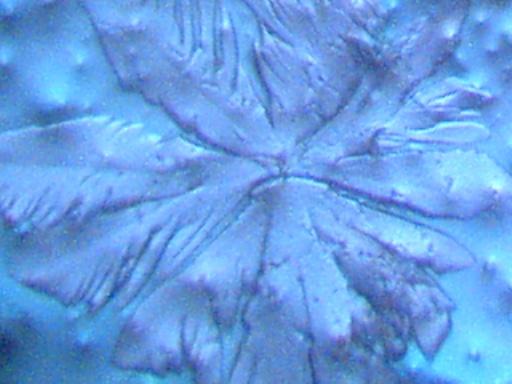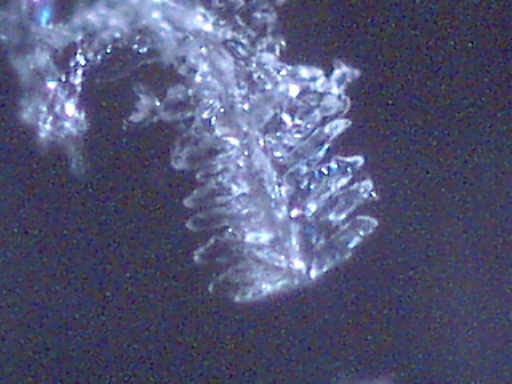
DENDRITE-IONIZER CRYSTAL UNDER EXAMINATION
I have recently been notified of an unusual crystal form that has developed within an ionizer. A detailed description of the circumstances of occurrence is presented below by the sender of the sample; the original report was submitted on July 20, 2003. I have placed the material under the microscope and have taken several microphotographs at a magnification of 200x; these photographs also are presented below. The crystals have a unique branch-like, or dendritic structure, and they are highly soluble in water. Any individual with additional information regarding the nature of this sample is welcome to contact me at cec102@usa.com. Appreciation is extended to the sender for the efforts that have been made to make this information available to the public for further examination.