Acids to the Demise
Clifford E Carnicom
Apr 07 2024
An additional fundamental aspect of the Cross Domain Bacteria (CDB) (nomenclature, 2014) synthetic biology under study by Carnicom Institute (CI) has been defined. This is the existence and role of acid production by this xenobiotic, microbial life form. This progress is in place because of the recent identification of specific acids that are created within the CDB metabolism. This acidic nature, the ability of the CDB to disrupt iron function and induce clotting in human blood, and the formation of polymers in the body are defining characteristics of this synthetic biology. This combination of harm poses a tangible threat to human health and the future of our species.
The primary acid operative in the CDB culture is acetic acid. It has been known for some time that the CDB has an important acidic nature to its growth, but the identification of a specific acid has been a fleeting prospect for too long. The CDB culture process exhibits complex biology and chemistry. To identify this particular acid required a phase change polymer formation (described previously) as well as five separate stages of distillation to arrive at a purified form of the acid. This specific identification will produce many benefits in future mitigation (and termination?…) understanding, especially when combined with the blood and polymerization damage already known.
On the surface, acetic acid might not appear to be of much consequence, as it has the common name of vinegar as we know. But from another viewpoint, an acid is an acid is an acid; strength and duration of exposure are both factors on par (ever notice holes in your pants a week after working with an auto battery?…)
The primary issues are that of interaction with other products from the CDB, as well as the stable maintenance of the acid-base chemistry of the body. There also remain many unknowns ahead, as each additional discovery adds further interaction study. We are speaking here of chronic exposure of unwanted acid(s) in the body, not of an occasional nutritional ingestion by choice.
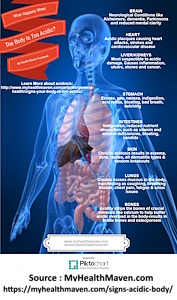
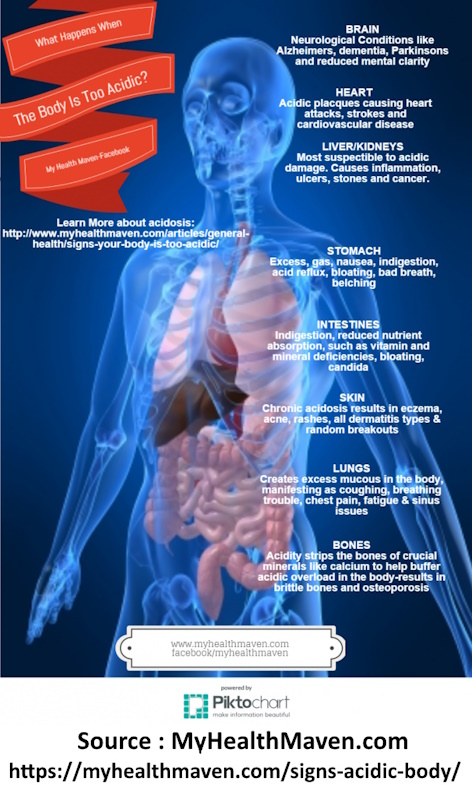
To get an overview of some of the human health issues at play with off-balance, internal acid exposure you might wish to investigate the site below. It presents a well rounded synopsis of the many consequences at bay. It may also be worthwhile to devote attention to the references that MyHealthMaven provides; acid-base balance of the body is hardly a trivial issue, even though certain parties often attempt and wish to make it appear so.
https://myhealthmaven.com/signs-acidic-body/
https://myhealthmaven.com/signs-body-acidic/
The above information will serve as a good introduction to the seriousness of the matter, and we can call the short version of this paper at an end here. There is no exaggeration in MyHealthMaven’s work or journey. For those with sufficient interest, let’s look at additional considerations that arise from the identification of a specific acid (actually, four acids are on the table already…)
___________________________________________________________
___________________________________________________________
First off, let’s explain how four acids come into the picture right away, not one. The four acids under discussion are:
1. acetic acid
2. hydrogen peroxide
3. iron and iron complexes
4. peracetic acid
Let’s learn how they arise in the picture.
1. Acetic acid has now been identified to exist in culture by a variety of methods. The fundamental methods used are that of titration, pKa determination, UV spectroscopy and pH measurement. Deductions and comparative candidates such as formic acid and citric acid were also at play, as well as conventional bacterial metabolism.
2. Hydrogen peroxide also meets the chemical definition of an acid. It is a relatively weak acid, but an acid nevertheless. Hydrogen peroxide was identified and discussed in a previous paper, along with the major ramifications of hydroxyl free radical production and subsequent polymer development.
3. Ferrous and ferric iron solutions can be or are acidic in their own right. The pH of a solution of ferrous sulfate has been measured directly at approximately 3.0.
4. Peracetic acid is an unexpected deductive consequence of the previous conditions that have been discussed in combination with the now identified existence of acetic acid. The conditions necessary for the formation of peracetic acid exist within the culture environment. These include ferrous iron, hydrogen peroxide, the hydroxyl radical and acetic acid. It is a safe precaution to assume that it can or does exist in the culture environment, and hence the human body as well. This is an additional concerning development, as this particular acid has a toxicity level that operates in the 4 ppm range, an extremely low and difficult to detect concentration level. It is a extremely hazardous acid to human health, even at minuscule levels.
These acids combine to deepen the case for detrimental effect to the human body, and it is fair to presume that a chronic exposure to excess acid from the CDB does exist. All issues brought forth by MyHealthMaven mentioned above are on the table as a consequence of this paper.
I will now list additional concerns that arise from the composite acidic nature of the CDB synthetic biology that is firmly established and in existence:
- Acetic acid, in combination with Fenton’s reaction (described previously) can further affect the production of free radicals and consequently can further affect the polymerization of vinyl functional groups.
- Acetic acid and peracetic acid can irritate the skin, eyes and respiratory system.
- The existence of acetic acid in combination with synthetic blood (known to be produced by the CDB) and peracetic acid (presuming it exists) is expected to further affect polymerization and human health in a complex manner.
- Acetic acid production is a known method (ATP) to produce energy by certain acetic acid bacterial species.
- Acetic acid can irritate tissues.
- Excess acetic acid or acid in the body can stress or overwhelm the liver, leading to conditions of acidosis.
- Kidneys help to regulate the acid-base balance in the body; an imbalance introduces additional stress on this organ.
- The CDB culture is known to contain an alcohol functional group. The oxidation of alcohol (e.g, by hydrogen peroxide) can lead to the formation of acetic acid and ketones.
- There is a relationship between diabetes and excess acid in the body. Diabetes produces ketones, which are acidic. The failure to adequately remove this acid can also lead to acidosis.
- The combination of ketone and acetic acid production can lead to: cellular stress and damage, inflammation, and organ dysfunction (e.g., kidney).
- Excess acid in the body can affect enzyme form and function.
- Excess acid in the body can affect protein form and function, including hemoglobin, and consequently diminish the oxygen carrying capacity of blood.
- Excess acid in the body can lead to increased cellular aggregation and clumping, including blood.
- Excess acid in the body can affect cell membranes, affecting nutrient intake and waste removal.
- Excess acid in the body can affect ion gradients in the body (e.g, electrolyte transfer), subsequently affecting nerve and muscle function.
- Excess acid in the body can activate an immune response.
- Excess acid in the body can lead to inflammation and tissue deterioration.
- Excess acid in the body can damage kidney cells, induce scarring of tissue, and diminish the filtering capability of the kidney.
- Excess acid in the body can promote the formation of kidney stones.
- Excess acid in the body causes the kidneys to work harder to reduce the acid load.
- Long term exposure to low levels of acid may show effects on par with shorter time higher concentration damage.
- Exposure to peracetic acid, even at low concentrations, can lead to respiratory distress, lung and digestive tissue damage, and fluid buildup in the lungs (cautionary presumption of existence).
- Exposure to peracetic acid can detrimentally impact hemoglobin form, function, and oxygen carrying capacity.
In closing, I will re-emphasize the following discussions in previous papers:
1. Knowledge of the specific biology, chemistry, and biochemistry of the CDB synthetic biology inevitably leads to the development of numerous (i.e, scores, without hesitation) mitigation strategies to inhibit or terminate the harm, damage and threat from the CDB synthetic biology.
2. The health and nutritional professions, of all orders, are responsible for serving the public health and welfare. These same professions are responsible for providing the information and protocols for mitigation (or termination) strategies. These strategies are to be developed in a manner that provide accessibility and affordability to all. Drugs and money motives do not qualify here. Please see Four Mechanisms and a Future for Mitigation (or Termination) (Mar 2024).
3. It is best to work at the origin of a problem rather than on the tail end.
Sincerely,
Clifford E Carnicom
Apr 07 2024
born Clifford Bruce Stewart, Jan 19 1953