MORGELLONS : A THESIS
Clifford E Carnicom
October 15 2011
Edited Dec 01 2011
Edited May 10 2013
Note: I am not offering any medical advice or diagnosis with the presentation of this information. I am acting solely as an independent researcher providing the results of extended observation and analysis of unusual biological conditions that are evident. Each individual must work with their own health professional to establish any appropriate course of action and any health related comments in this paper are solely for informational purposes and they are from my own perspective.
Abstract:
A substantial body of research has accumulated to make the case that the underlying organism (i.e., pathogen) of the so-called “Morgellons” condition, as identified by this researcher, is using the iron from human blood for its own growth and existence. It will also be shown that the bio-chemical state of the blood is being altered in the process. The implications of this thesis are severe as this alteration affects, amongst other things, the ability and capacity of the blood to bind to oxygen. Respiration is the source of energy for the body.
This change is also anticipated to increase the number of free radicals and to increase acidity in the body. This process also requires and consumes energy from the body to take place; this energy supports the growth and proliferation of the organism. The changes in the blood are anticipated to increase its combination with respiratory inhibitors and toxins. The changes under evaluation may occur without any obvious outward symptoms. It is also anticipated that there are consequences upon metabolism and health that extend beyond the functions of the blood. This change represents essentially a systemic attack upon the body, and the difficulties of extinction of the organism are apparent. Physiological conditions that are in probable conjunction with the condition are identified. Strategies that may be beneficial in mitigating the severity of the condition are enumerated.
This paper will present this case progressively, and it will build upon the information that has been presented in previous papers. The paper will sequence through the following topics of discussion:
1. A Brief Introduction to the Chemistry of Iron
2. Beginning Observations
3. Qualitative Chemical Analysis
4. An Introduction to Bonding : Ionic, Covalent, Polar Covalent and Coordinated Covalent Bonds
5. The Structure of the Heme Molecule and the Role of Ligands
6. Qualitative Chemical Analysis of the Oral Samples : Two Methods to Verify the Existence of Ferric Iron
7. A Method to Extract the Oxidized Iron within the Filament Growth Structure
8. A Discussion of Ligands
9. Spectral Analysis of the Blood and a Comparison to the Growth Spectrum
10. Methemoglobinemia and Hypoxia
11. Ionization and Bond Disassociation Energy : The Cost of Oxidation
12. Bacterial Requirements for Iron in the Blood
13. The Oral Filament and Red Wine Reaction Resolved
14. Some Health Implications; The Value of the Holistic Approach to Medicine
15. Identification of physiological conditions that are in probable conjunction with the condition.
16. A Proposed Spectral Analysis Project
17. A Review of Proposed Mitigation Strategies
As we continue with our discussion, there will be three different general approaches that will be used in a combined sense to reach the conclusions that have been stated above. The first of these will be direct observation, the second will be qualitative chemical examination, and the last will be the use of spectral analysis and analytics. A synthesis of each approach will give us the understanding of the situation that we require. Let us begin with some discussion on the chemistry of iron and then follow with a few of the qualitative iron tests that are helpful in the methods that have been developed.
1. A Brief Introduction to the Chemistry of Iron:
Let us start with an introduction to iron. Iron exists in three primary forms in nature, the first in its elemental form with no net charge, and the other two as compounds, known as ferrous and ferric compounds. It is these latter two states of iron that will be of interest to us in terms of human biochemistry.
Ferrous compounds involve iron in a charged state, known as Fe2+, and ferric compounds involve iron in the valence state of 3, or Fe+3. The term valence refers to the number of electrons lost or gained in a chemical reaction. For example, a loss of two electrons from an atom will leave the atom in a charged state of +2. A charged atom or compound is called an ion or ionic compound, respectively.
Why is this important to us and why should we learn about the chemistry of iron? It is because iron is in our bodies and it is absolutely crucial to our lives and our health. The charged state of the iron in our bodies and our blood is of the utmost importance in understanding the changes to human health that are occurring.
Now let us start focusing upon the iron in blood. Your blood needs iron to function. Not only does your blood need iron to function but it needs the iron to be in a particular state for your blood to work properly. The iron in your blood must be in the ferrous form, or the Fe2+ in order to bind to oxygen1,2,3,4,5. If it is not in this state (e.g, ferric iron or Fe3+), it will not bind to oxygen and human health will suffer. You are not thriving in an energetic sense if you do not have the proper oxygen content within your blood.
Hopefully we understand that the state of the iron in our bodies is not a trivial affair and it is in our interest to become educated on the matter. It is the very path that I have chosen in this research and the implications of these studies are profound.
Now let us talk, in a general sense, about what causes iron to change state. What for example, would cause iron in the elemental form (Fe) to go to the Fe2+ (charged) state, or for that matter, from the Fe2+ state to the Fe3+ (further charged) state? It is here that we introduce and explain the term of oxidation. As a familiar example, when something rusts, it is being oxidized. What it means, in a more descriptive sense, is that a chemical reaction is taking place and that electrons are being removed from an atom or substance. Formally speaking, oxidation refers to the process of losing electrons. Oxidation increases the charge state of the atom or ion, because as an electron (i.e, negative charge) is removed, the atom, ion or substance becomes more positive as a result. A typical example of oxidation is the change of iron from the Fe2+ state (i.e, ferrous) to the Fe3+ (i.e., ferric) state mentioned above.
Below are some photographs that show testing of the iron ion in varying oxidation states, ie., Fe2+ and Fe3+ with the use of some specialized chemical reagents. One of the factors that is important in the qualitative tests that we are doing is that of color; color is an extremely useful tool for determining the existence of metals in solution and for the chemical state that they are in.
This set of photographs shows a solution of what is called “liquid iron”, essentially a solution of a ferrous salt (with some minor impurities) that is used in gardening applications. This ferrous solution is formed from a representative iron salt with the iron in the Fe2+ oxidation state. One of the important characteristics visually of the Fe+2 iron is the greenish tint that often accompanies the Fe2+ iron oxidation state. The photograph to the right shows the addition of a chemical (1,10 phenanthroline) that is very sensitive to the presence of the Fe2+ ion, and it turns the solution red in combination with the ion. The use of this chemical is a valuable and sensitive qualitative method to determine the existence of the Fe2+ ion.
This set of photographs is provided to demonstrate the variability of color as well as its value and importance. The photographs above show a freshly dissolved solution of ferrous sulfate. When the ferrous sulfate is dissolved in water it will ionize (separate into ions of Fe2+ and (SO4)2-). It will also generally turn light green in color but this example lacks the stronger green tint shown in the set to the left. Colors can easily be influenced by concentrations and impurities. A separate solution made previously demonstrates a stronger green tint that is characteristic of the Fe+2 ion; this particular one does not. The use of 1,10 phenanthroline reagent resolves the issue very clearly, however, as the characteristic reaction to produce the bold red color in combination with the Fe2+ ion is evident. This example demonstrates the value of approaching the problem from more than one perspective, such as with the use of color, chemistry and spectral analysis for a more comprehensive assessment of the situation.
This set shows an analogous qualitative chemical test for the presence of the Fe3+ ion solution. This particular solution is that of ferric chloride. There is an expected similarity in color between various ferric salts, as the ionic form of iron is the agent responsible for the color. A distinctive feature of the Fe3+ ion in solution is that of a yellow to brown color.
This photo also shows the use of a different, but equally important, reagent that is used to detect the presence of the Fe3+ ion in solution. The chemical used in this case is that of sodium thiocyanide. Even though this reagent also produces a bold red color, this test and the one mentioned above using 1,10 phenanthroline are entirely separate and unique from one another, and are only valid for the particular ion of each test.
The value of the tests shown above are threefold:
1. First we have a sensitive qualitative method of identifying the existence of specific iron ions, i.e., Fe2+ and Fe3+ in solution6. These tests can also be extended in combination with a spectrophotometer to provide concentration levels of the ions, if required7.
2. If the test succeeds, we know that the iron states are present in an ionic form within the solution. If the test fails, it does not mean that Fe2+ or Fe3+ are not present, it only means that they are not present in ionic (i.e, disassociated) form. It is possible that the iron could exist in a different form (e.g., bound within a molecular compound) than ionic, and the test would not show this fact. This distinction will become important in later testing procedures that are described.
3. Regardless of individual variations, there is a clear and distinctive difference between the greenish tints associated with the Fe2+ ion and the yellowish and brown tints that result from the Fe3+ ion. This distinction will also become important in later testing.
2. Beginning Observations:
Let us now switch over to the course of direct observation. Many of us may recall that certain culture growth trials were discussed in an earlier paper entitled “Morgellons : A Discovery and a Proposal8. In that paper, conditions and circumstances that both increased and inhibited the rate of growth of the organism were discussed. A section of that paper again is relevant again with direct observation, as shown below, in combination with the color characteristics of iron discussed above. Direct observation essentially indicates to us that the organism is able to utilize and absorb iron in the Fe3+ state. Let us discuss further why this is the case.
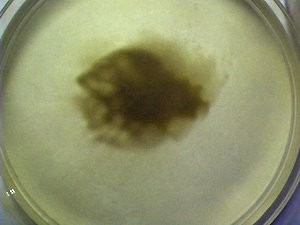
This photograph shows a culture that has just been started. The process of starting a culture with this method requires only a single drop of the culture solution. The culture solution is prepared by subjecting the pulverized and dried filaments of previous growth to sodium hydroxide in solution and heat to the boiling point. The culture medium has ferrous (Fe+2) sulfate and hydrogen peroxide added to it as described in the paper referenced. This chemical reaction that takes place will again be described in more detail below.
This photograph shows the state of the culture growth after a few days have elapsed. The dark brown color characteristic of the ferric (Fe+3) oxidized iron within the organism growth is visible. The organism is absorbing the nutrients that have been provided in the culture medium. In this case, the Fe+2 ion originally introduced into the solution was oxidized by the hydrogen peroxide (Fenton’s reaction) to produce the Fe+3 iron state. The organism is able to nourish itself from this oxidized state of iron and it imparts the characteristic color of the iron (Fe3+) oxidation state within the growth of the culture.
In order to understand the results of the photographs above, it is helpful to describe a chemical reaction, called “Fenton’s reaction” that was discussed in the former referenced paper9. Fenton’s reaction involves the combination of iron in the Fe2+ state (in this case, ferrous sulfate) and hydrogen peroxide. The reaction is as follows:10
Fe2+ + H2O2 Fe3+ + OH. + OH−
Fe3+ + OH. + OH−
This reaction was established in the following manner: A starter culture of the underlying organism was introduced into distilled water. A few drops of a ferrous salt solution, namely ferrous sulfate was introduced into the culture. This was followed by a few drops of hydrogen peroxide. It has been learned that this culture medium rapidly accelerates the growth of the culture. The result of the combination of the iron in the Fe2+ state with hydrogen peroxide produces three things:
1. Iron ions in the ferric state, or Fe3+.
2. The hydroxide ion (not a radical), OH-
3. The hydroxyl radical, a highly reactive free radical.
Notice that none of these three developments were dependent upon the culture; Fentons reactions would have taken place irregardless of the introduction of the organism. What we do know from the reaction, however, is that the iron is oxidized to the Fe3+ state and becomes immediately available to the organism along with the hydroxyl radical. The paper mentioned discusses some of the ramifications of this combination with respect to health. Not only does the oxidation takes place, but we see that the organism is directly able to utilize the iron in this oxidized state (Fe3+) for its growth and sustenance.
This provides our first link in understanding the role of oxidation of iron in our body and its relationship to the growth of the organism. All of the conditions described for the controlled petri dish trial are also to be found to occur within the human body.
3. Qualitative Chemical Analysis:
There are chemical tests which can be performed to determine the existence of substances, particularly those in ionic form. These tests are valuable in that they are relatively simple and yet they can provide crucial information as to the existence of a metallic ion, for instance, without providing quantitative or concentration levels. Examples of this include the determination of the existence of the iron ions (both ferrous and ferric), copper ions, sulphate ions, chloride ions and others11,12,13. It is important to understand that the tests being described in this section are for ionic forms only, i.e, they are in a disassociated form in solution. A negative test does not mean that the element in some form does not exist, (.e.g, bound in a molecular form); it only means that it does not exist in an ionic form. This distinction will become important to us as we proceed later with additional laboratory procedures.
An excellent example of a qualitative test for the presence of ionic forms of iron has already been described in the earlier section of this paper, entitled An Introduction to the Chemistry of Iron. In this case, as described, certain reagents were used to positively identify the presence of the Fe+2 and Fe+3 ions in known solutions.
Now let us apply these methods to the questions at hand, which are twofold:
1. Does human blood in solution contain iron ions? We know that blood contains iron, so it will be of interest to examine if it exists in ionic form.
2. Does the culture solution (as developed from oral filaments characteristic of Morgellons) contain iron ions?
Let us discuss the first question, i.e., does blood contain iron in ionic form? If so, is it in the Fe2+ state, or the Fe3+ state, both, or none? We can answer this question with the application of the same reagents mentioned earlier, 1,10 phenanthroline for the test of Fe2+ ions and sodium thiocyanide for the testing of Fe3+ ions.
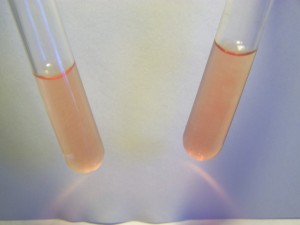
The results in both cases are negative. This means that human blood does not show the existence if iron in ionic form, either Fe2+ or Fe3+ within it. It does not mean that blood does not have iron within it, for we know that it does. But in what form does it exist then? If it is not ionic, is the iron bound in some way? If so, what is it bound to? How do we know what state it is in (Fe2+ or Fe3+) if it is bound to something? These are some of the questions before us. The answers to these questions will become important to us in our understanding of any changes taking place to the blood and they will become equally relevant in our tests of the culture solution based upon oral filament growths. This result also raises the problem of how do we go about qualitatively testing for iron in the blood as we have now learned that the direct ion testing approach is not sufficient.
As we proceed, please keep in the forefront that our problem is to approach the question of how the state of oxidation of blood is affected by the Morgellons condition.
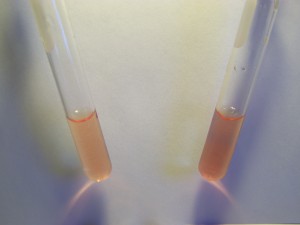
Now let us test the culture solution in the same way: The preparation of the culture solution can be described in detail at a later time; this has been summarized to some degree in previous papers.


The results are again in both cases negative. This tells us correspondingly, that the culture solution does not contain iron in the ionic form (Fe2+ or Fe3+), at least to the degree of sensitivity of the tests. Once again, it does NOT mean that the culture solutions do not contain iron, only that if it is present that it is not in the ionic (disassociated) form. The issue, therefore, must provoke our testing methods further and the question of iron binding to other molecules, even if in an oxidized state (Fe2+ or Fe3+), rises to importance.
4. An Introduction to Bonding : Ionic, Covalent, Polar Covalent and Coordinated Covalent Bonds:
Soon we must educate ourselves further on how iron exists within the blood. Before that occasion, however, we must also spend some time talking about the various methods that atoms use to bind together to form molecules and compounds. Much of what happens in chemistry is in some way related to bonding and it is helpful to have at least some background on the subject. Ultimately, the knowledge is crucial to our understanding and determination of how the oxidation state of blood is altered.
Within conventional chemistry, there are two forms of bonding of atoms that occur: ionic and covalent. Ionic bonding means that electrons are transferred from one atom to another. Covalent bonding means that the electrons are shared between atoms. Bonding is important because the physical properties of a substance are generally entirely different depending upon the type of bonding that exists. Therefore, if you know what type of bonding is occurring within a molecule or substance, you can likely determine quite a bit about the physical properties and behavior of the substance. In our case, this is not an academic exercise and we do not have a choice; we need to learn as much as we can about the properties of the blood and how it interacts with the rest of the body. Science is more meaningful is we can give value and application to our studies and in our current situation, our very lifeblood and welfare depends upon this pursuit. Consider taking some time to learn about the chemistry and biochemistry that is involved here and we will all be the better for it.
The following are simple illustrations of both ionic and covalent bonding:
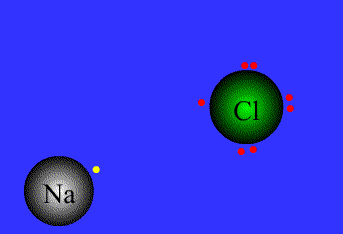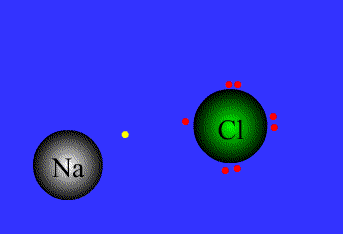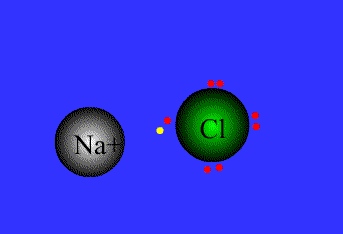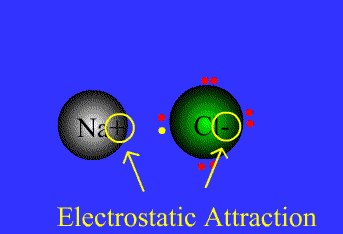
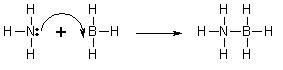
An example of ionic bonding.
The transfer of electrons characterizes this bond form.
Source: Northeastern Oklahoma A&M College
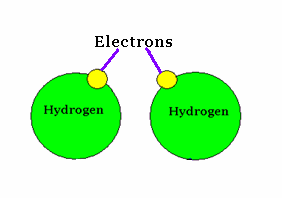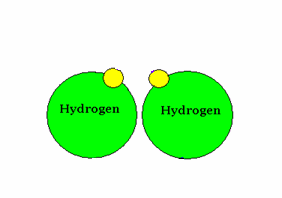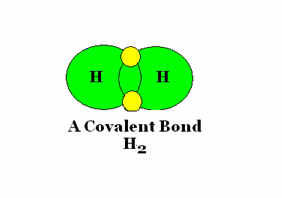
An example of covalent bonding.
The sharing of electrons characterizes this bond form.
Source : Mr. Wolgemuth GHHS Science Web Site
Next, a brief word on polar covalent bonding: Polar covalent bonding is a variation on the covalent bonding theme shown above. In the example above on covalent bonding, the forces on the electrons are symmetrical. When different types of atoms join together(as shown below) vs. atoms of the same type (as in the two hydrogen atoms shown above), the forces between the electrons are not necessarily symmetrical. This asymmetry of forces between shared electrons is referred to as a polar covalent bond. A simple example of polar covalent bonding, i.e., the water molecule, is shown immediately below. These three types of bonds: ionic, covalent and polar covalent cover most of the ground of conventional and introductory discussions of bonding of atoms within chemistry.
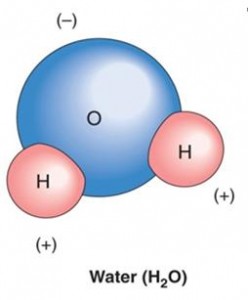
An example of polar covalent bonding.
The asymmetric sharing of electrons and unequal distribution of charge characterizes this bond form.
Source : Zendarie : Biology One Step At a Time
[http://zendarie.com/2011/chemical-bonds/ : Server Not Found 404 12/13/15]
We, however, in our journey of understanding the nature of iron bonding within blood, are not allowed to stop here. We will find that the three bond types above do not tell us what we need to know about the way in which iron is bonded, or “held” within the blood. There is indeed a fourth type of bonding that we will introduce, and we will find that it is different, unique, interesting and important to know about when it comes to understanding what is happening within our blood. The bond type that is pertinent to our need to know is called a “coordinated covalent bond“.
The coordinated covalent bond is an interesting animal, as it does not fit in very well with any of the conventional explanations of bonding listed above. What has caught my interest is that the coordinate covalent bond is not introduced in the forefront of chemistry education, but from my vantage point, it can easily end up being a most important form of bonding to know about. It seems to me that one of the easiest ways to attempt to visualize a coordinated covalent bond is to imagine at atom being “held” or “suspended” or surrounded by electrons, the forces of those electrons keeping the bond in place. Let us get the formal definitions, and then go to work with an image that can help us to understand this unique form of bonding. Here are three definitions to work with:
To start:
“A coordinate covalent bond is a covalent bond in which one of the bonded atoms furnishes both of the shared electrons”13.
Also:
“A particular type of covalent bond is one in which one of the atoms supplies both of the electrons. These are known as dipolar (or coordinate, semipolar, or dative) bonds.”14
And:
“A covalent bond occurs when one atom contributes both of the shared pair of electrons. Once formed, there is no difference between a coordinate bond and any other covalent bond.”15
And lastly, for the person in greater need, here is a more detailed online definition16 and description of the coordinate covalent bond.16
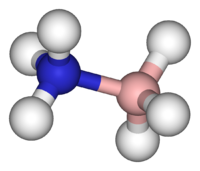
An example of coordinate covalent bonding.
This is called a Lewis diagram and it shows the arrangements of the electrons in the outer shell of the atom and how they are “shared” or coordinated.
Source: New World Encyclopedia: Covalent Bond
A three-dimensional model of the coordinate covalent bond shown to the left..
Source: New World Encyclopedia: Covalent Bond
Now let us try to give more meaning to what the coordinate covalent bond entails. The images above depict one of the simpler presentations of a coordinate covalent bond. Both images are different views of the same bonding process. What the picture shows on the left is that instead of one electron being shared by each atom (in this case, Nitrogen and Boron) to form a shared pair, BOTH electrons are donated by the Nitrogen atom and none by the Boron atom to form the bond. The end result is the same as in a regular covalent bond, but the process by which the bond was achieved differs from a normal covalent bond. The reason that this type of bonding is important is that many types of new and fundamentally important “complexes” or chemical structures can be formed. Our blood structure is one such example. Many of the complexes that are formed in this way involve the bonding of a metal atom (e.g, iron) with surrounding molecules, and this leads us directly into our discussion of the blood and the hemoglobin (or heme) molecule. The formation of what are called coordination complexes or coordination compounds, very often with metals at the center of the structure, is one of the most important practical branches of chemistry. It is necessary for us to understand coordination complexes in order to understand how the iron in our blood bonds to oxygen. And so now that we are in the thick of it, on we go…
5. The Structure of the Heme Molecule and the Role of Ligands:
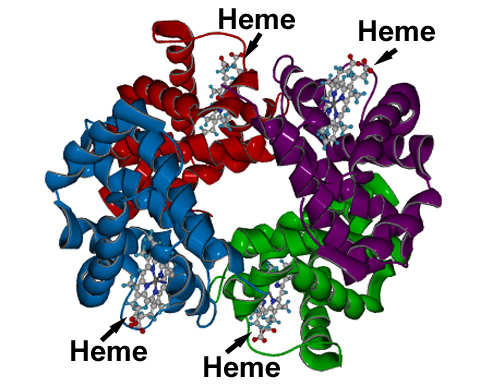
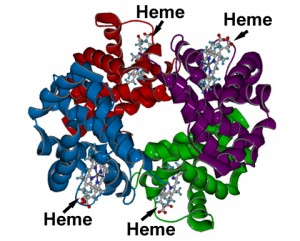
We are now in position to become more familiar with the detailed structure of blood. Our interest will be centered on hemoglobin, and in even greater detail, upon what is known as the heme molecule. Hemoglobin is an iron containing protein within red blood cells. Hemoglobin is the molecule that transports oxygen.17 It is the iron of hemoglobin that binds to oxygen18. Heme is one of four subunits within hemoglobin. Each heme group has an iron atom at its center, and therefore each hemoglobin molecule can bind to four molecules of oxygen (O2).19 Our primary interest will be in the heme group, as it is where the oxygen carrying capacity exists. Here are a couple of images to familiarize the reader with the overall structure of hemoglobin and the heme group. Subsequently, we will examine the heme group in even greater detail along with the bonding process.
A generalized model of the hemoglobin molecule.
Notice the four subunits of heme within the hemoglobin molecule; this is where the iron atom exists that can bind to oxygen.
Source: Washington University, Department of Chemistry
A closer view of the heme group.
The iron atom(orange) resides in the center of the heme group. The oxygen (O2) molecule is in red above the iron atom. We will examine this structure and bonding process in greater detail below
Source : Wiley : Biochemistry
The type of bonding that allows the heme group to exist and to bind iron to oxygen as shown above is the coordinated covalent bonding that has been introduced previously. This type of bonding allows the formation of a multitude of metal complexes, and the heme group is an example of one such structure that incorporates a coordinated metal complex. These metal complexes and the unique type of bonding they incorporate are have a special importance in biochemistry and in blood. Let us now look at the heme group in even greater detail to understand the molecular structure further:
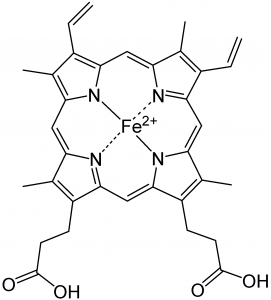
The heme group, consisting of an iron atom in the Fe+2 state, surrounded by four nitrogen atoms bound with coordinated covalent bonds. The iron must be in the +2 state to be able to bind to oxygen..
Image source: Wikipedia
A three-dimensional model of the heme group, with the iron (II) atom at the center surrounded by the four nitrogen atoms. This type of structure is known as a porphyrin. One of the best known porphyrins is heme, which is the pigment in red blood cells.
Source: Argus Lab
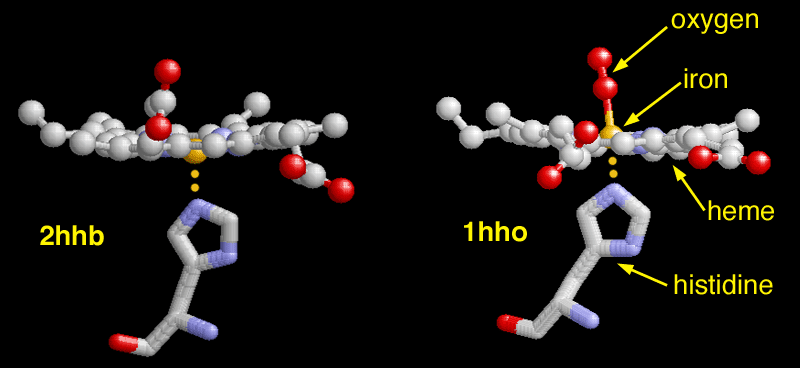
The dexoxygenated heme molecule (model) shown
with oxygen atoms (red) removed (left) and attached (right).
The heme group consists of an iron atom in the center of a ring structure, termed a porphyrin. The porphyrin includes the central iron atom in the +2 oxidized state and is surrounded by four nitrogen atoms with coordinate covalent bonds. The upper two photographs of this sections show this structure in both a planar view and a three-view. The coordinate covalent bonds, as discussed earlier, allow the transition metals such as iron to bind to a host of varying molecules. This type of structure is also that known as a chelate, where a central atom is bound to surrounding molecules or structures (termed ligands). A great variety of molecular structures with the transition metals can occur with this unique and more complex bond type, i.e., the coordinated covalent bond.
The lower photograph shows two additional aspects of the heme molecule and the bonds that it makes within. These include the histidine (an amino acid) structure and the oxygen molecule. The oxygen molecule is at the heart of the discussion here. The left photograph within the pair shows the oxygen molecule removed from the heme group and the right photograph within the pair shows the oxygen bound to the Fe2+ atom. The iron must be in the Fe2+ state for the oxygen to bind; transport of oxygen is a vital and crucial function of the blood within the human body. If the iron in the blood is changed to the Fe3+ state, the bonds to oxygen are broken and the blood is then known as deoxyhemoglobin. The primary cause of change in the oxidation state of an atom is from an oxidizer; some of the best known oxidizers include the hydroxyl radical, ozone, peroxides and bleaches20. Oxidizers exist with the human body to some level naturally. There is a body of evidence available in the literature that will demonstrate that excessive exposure to oxidizers within the body can be detrimental to human health. Oxidizers produce free radicals, which are highly reactive molecules that can “wreak havoc within the living system”21. Some of the most important free radicals in biology are the superoxide anion (O2–), peroxide (O2-2) and the hydroxyl radical (OH)22.
It will become apparent that the change in oxidation state of iron from Fe2+ to Fe3+ in sufficient numbers within the blood is generally detrimental to the blood and human health. It will become equally apparent that this change is especially beneficial to the growth of the organism and filamentous biological growth structures that are characteristic of the Morgellons condition.
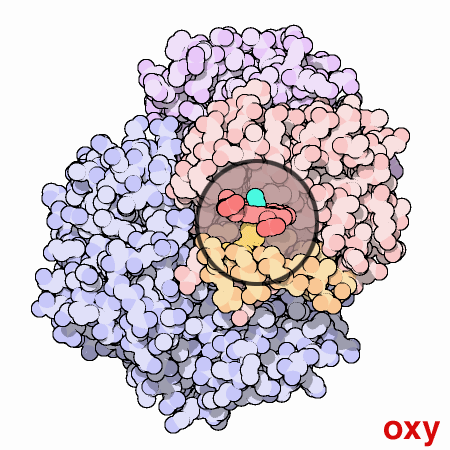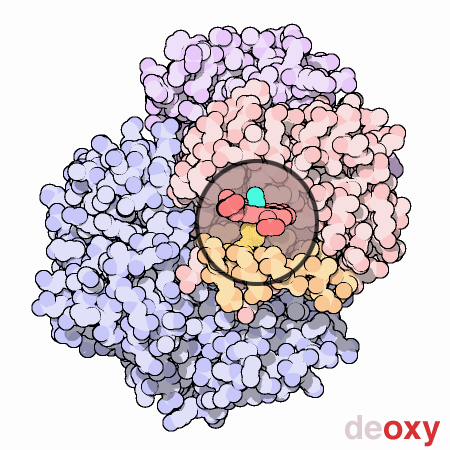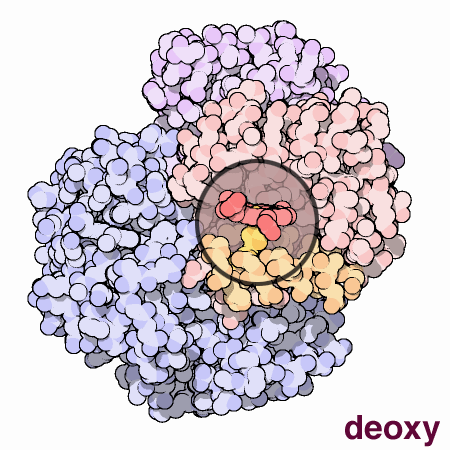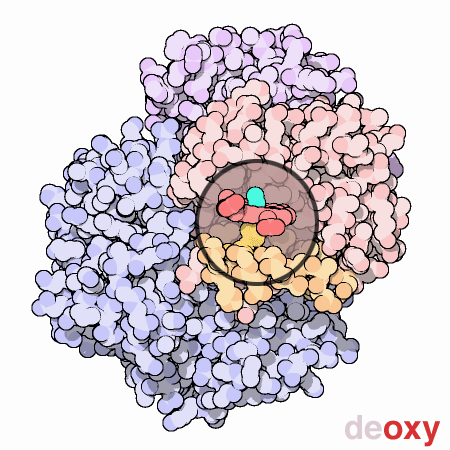
An animated view of the change between the oxygenated and deoxygenated states of the blood. Correspondingly, this results is a shift between the Fe2+ oxidation state of iron and the Fe3+ oxidation state of iron in the blood.
Source : Protein Data Bank
http://pdb101.rcsb.org/learn/resource/oxygen-binding-in-hemoglobin-gif
6. Qualitative Chemical Analysis of the Oral Samples ; Two Methods to Verify the Existence of Ferric Iron:
We are now in a position to better understand and interpret the results of more direct laboratory analysis. It will be found that there is essentially little difference between the direct human filament samples that are under examination and those that result from the culturing process demonstrated repeatedly on this site. At this point we will deal directly with human oral filament samples as the chemical reactions that are common to both forms are now better understood.
It has long been observed that extended exposure (e.g., three minutes +/-) of the oral gums to red wines produces in many, if not most, individuals a purplish filamentous mass than can be expelled and further analyzed. This discovery is fully credited to Gwen Scott, N.D. as originally reported several years ago23,24. It is claimed by some individuals that this mass is of a precipitate form and that it is a natural reaction between red wines and saliva. The reaction referred to is valid and has been studied as well. However, the statement as it has been made is entirely false as it refers to the samples under examination. The sample under examination is of a filament form, and it is not a precipitate. The sheer volume of material that can be expelled, let alone the examination of the material, is sufficient to dispel the false and diversionary claims25.
The chemistry of this rather dramatic reaction of filament production and coloration has, prior to this study of the last several months, been unknown. This is no longer entirely the case, and the subject will be introduced again later in this paper. For now, suffice it to say that a most significant chemical reaction and filament production does take place, and the discovery can be regarded as serendipitous and fortunate to the studies that have been made.
Given that such a reaction and production of mass does occur, this study has now examined the material in greater depth from a qualitative chemical perspective. It has also been known for some time now that the filaments do break down and undergo chemical transformation when exposed to a solution of sodium hydroxide (lye) and heat26.
An oral sample filamentous mass produced from extended exposure of the mouth gums to red wine. The sample has been repeatedly rinsed and decanted in distilled water. The purplish color and microscopic filaments are characteristic of the sample.
The oral sample after it has been subjected to a process of alkalizing, heating and filtration. The sample is treated with sodium hydroxide (lye) in solution and heated to the boiling point. The solution is then filtered and produces the colored solution above. Please recall that the color of the ferric ion (3+) is usually yellowish to brownish and that the color of the ferrous (2+) ion is generally more greenish in color. This result of this process indicates that the ferric (3+) iron form is a candidate for further investigation in this qualitative analysis.
The photographs above show the original sample (to the left) and the sample after processing with alkali, heat and filtration (right). The solution on the right is also suitable for spectrophotometric analysis, as shall be discussed later. At this point, we will be concerned only with qualitative chemical reactions.
It is already known that the sample in the solution form prepared immediately above fails a test for the existence of Fe2+ and Fe3+ ions. This has been shown with similar results for the culture form of this study earlier in this paper. This result does not mean that iron does not exist in the solution, only that it does not exist in disassociated ionic form. The reason that the effort has been expended to understand the various types of chemical bonding is that because unless we know in what form a substance exists in solution we may not be able to detect it with common testing methods. This is the reason that an understanding of coordination complexes and coordinate covalent bonding is so essential; we must press the problem further and examine all options with respect to the possible existence of iron forms within the solution. The following three factors are thought to be relevant in the examination of the reaction of the oral sample solution with a copper sulfate solution:
First:
One of the types of chemical reactions is called a single displacement reaction. In a general way, this reaction has the form28:
A + BC -> B + AC
or
A + BC -> C + BA
and if A is a metal, A will replace B to form AC, provided A is a more reactive metal than B.
Second:
Another relevant topic here is what is called the activity series of metals. Some metals are more reactive than others, with water or acids and the activity series of metals lists that reactivity in a tabular form. For example, potassium, calcium and sodium are highly reactive metals with water, iron and nickel are moderately active, copper and silver are of very low reactivity, and gold and platinum are inactive. Here is an example of an activity series table27:
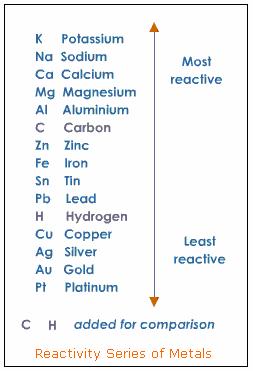
Source:
http://www.tutorvista.com/content/science/science-ii/metals-non-metals/reconcept-series-metals.php
It will be found that a metal higher on the list will replace a metal that is in ionic form and is lower in the list.
Third:
Another helpful known reaction is that iron ions (+2 and +3 states, respectively) in solution with sodium hydroxide will form ferrous (+2) hydroxide, a green precipitate, (Fe(OH)2) and ferric hydroxide, a brown precipitate, (Fe(OH)3) respectively.
The first chemical reaction that becomes of interest to study is the oral sample solution above when mixed with copper sulfate. It will be found that a reaction does occur, and the reaction is that a brown precipitate forms. This indicates that we are likely to have formed ferric hydroxide and this gives us another hint that we may be encountering iron within a +3 oxidation state within the original solution. The issue is complicated, however, by the fact that we know the iron is apparently not in ionic form. This would suggest that we are dealing with iron in a coordination complex of some type, where the iron is bound to an unknown ligand. There are still uncertainties in this problem, but it appears that the copper sulfate is somehow a factor in releasing the iron from a complex form (presumably affected by the activity series above) so that it can combine with the hydroxide ion to form ferric hydroxide. A proposed reaction is somewhat akin to the form:
Fe+3X + Na+ + OH– + CuSO4 + H2O -> Fe(OH)3 + Cu2+ SO42- + Na+ + H2O + X
where X is an unknown ligand that is attached to the iron ion. The resulting reaction has been tested further for copper and sulfate ions, respectively, and the results are positive and are therefore consistent with the above reaction.
An alternative proposed reaction is of the form:
[Fe(H2O)6]3+ + Na+ + OH– + CuSO4 -> Fe(OH)3 + Cu2+ SO42- + Na+ + 6H2O
in which case the ligand is water and involves coordination with the hydrated ferric ion.
A reaction of the oral sample solution with copper sulfate. A brown precipitate forms. A postulated identity of the precipitate is that of ferric hydroxide which contains iron in the 3+ oxidized state.
The proposed ligand form is one question that will need to be addressed further. In the interim, the important question to pursue is whether or not the precipitate is consistent with a ferric (vs. ferrous) hydroxide identity. To further test the proposal of ferric hydroxide as the precipitate, it will be found that ferric hydroxide is soluble in citric acid29. It is also known that ferrous hydroxide, when dissolved in citric acid, will turn the solution green (characteristic of the ferrous ion). Ferric hydroxide, when dissolved in ctiric acid will turn the solution to a brownish color (characteristic of the ferric ion). This test has been conducted and the result is positive, the precipitate is soluble in citric acid and the resulting solution is brownish in color. This further solidifies the proposed identity of the precipitate as that of ferric hydroxide.
A second method of verifying the existence of the ferric form of iron within the oral filament sample has been established30. This method involves the reduction of the Fe3+ iron state to the Fe2+ state using ascorbic acid, and then testing for the existence of the iron in the Fe2+ state. The steps of the process are:1
. The oral sample must be extracted with the red wine and the test conducted promptly; this is a time sensitive process that has been created.
2. The oral filament sample is rinsed repeatedly in clear water and decanted until the final mass is in clear distilled water.
3. The sample is treated with sodium hydroxide and’ heated to the boiling point and then filtered. The solution will be brownish in color as described earlier.
4. The solution is then treated with ascorbic acid. Ascorbic acid is a strong reducer (anti-oxidant).
5. The solution is then centrifuged.
6. The clear solution that results from centrifuging is separated and placed in a separate test tube.
7. A test for the Fe2+ ion is conducted using (1,10) phenanthroline. The test results are positive. This test demonstrates the reduction of existing iron in the Fe3+ state to the Fe2+ state.
In the reference cited, it will be noted that potassium ferricyanide is used in the reaction. This experiment introduces the role of another ligand that will be discussed in more detail later, and this is the cyanide ion. It will be seen that varying ligands form complexes with the transition metals; this is one of the many reasons we must familiarize ourselves with coordination chemistry and coordinate covalent bonds to understand how this organism interacts with the body.
A positive test for the existence of the ferrous ion after reduction by ascorbic acid using (1,10) phenanthroline.
7. A Method to Extract the Oxidized Iron from within the Filament Growth Structure
A third and final method of verifying the existence of the ferric form of iron within the oral filament sample has been established. In this case, the iron itself in an oxide form has been extracted directly from the oral filament sample using electrolysis. The method is both simple and effective. Many metallic salts, when subjected to electrolysis, liberate a gas at the anode and deposit the metal in pure form at the cathode31,32,33,34. Presumably this can apply to certain transition metal (e.g., iron) complexes as well and as evidenced by the results obtained. The method used is to apply a current to the oral sample solution directly. Voltage is applied at 6 volts for approximately 8 hours of time. The current in the solution has been measured at 0.7 mA. The electrolyte is sufficiently decomposed at the end of that period. The metallic compound is collected and heated and dried at the end of that period. It appears as though the bonds in the compound are quite strong as the compound is only mildly soluble in strong acids such as hydrochloric and sulfuric acids. The compound reacts vigorously to hydrogen peroxide as shown below in the video segment. The reaction shown involving the decomposition hydrogen peroxide to oxygen and water is an established and known catalytic reaction (in the same genre as Fenton’s reaction)35,36.
The results of all qualitative tests indicate that a ferric (3+) iron is a highly significant component of the growth structure and organism development. It is also presumed at this stage of the analysis that the iron exists primarily within a transition metal coordination complex with ligand structures that require further analysis and identification. An additional discussion on the ligand aspect of this study will follow.


Drying the metallic residue from the electrolytic processing of the oral sample.
The final iron oxide (ferric oxide) compound result obtained directly from the oral sample through electrolysis.
Ferric Oxide Compound and Hydrogen Peroxide Chemical Reaction:
This is a catalytic reaction that does not result in a change in the iron oxide form or mass.
Magnification approx 75x.
8. A Discussion of Ligands:
Let us talk about ligands for a moment. Remember that a coordination complex is formed with a metal atom at the center of the complex surrounded by atoms that donate electrons to form the coordinate covalent bonds. These donor structures are called ligands. The heme group that we discussed was a representative example of such a coordination complex, with the iron atom in the center of the ring with nitrogen atoms surrounding the iron. We also have a histidine (amino acid) group attached to the heme and then the oxygen molecules at a sixth position in the complex. We have also seen that the oxygen molecules can come and go within the complex depending upon the state of the iron in the center of the complex. Please review some of the images and discussion above if you would like to recall this discussion.
It now is becoming more apparent to us why we must understand the specific molecular structure of the hemoglobin molecules (especially the heme group within) and’ of the transition metal (notably iron) coordination complexes within the heme group. It is also equally important that we must learn more about the impact of “ligands”, as ligands are the atoms or structures that bind to the metal. Coordination chemistry seems to be a bit more involved than conventional chemical study as the bonding structures are highly varied and more difficult to predict. But the necessity exists here, for what binds to the iron (i.e., ligand) that has been altered (i.e., oxidized) is going to be extremely important in understanding the impact or predicted impact upon the body. For instance, the importance of this topic can be stressed with the following:
“Metal and metalloids are bound to ligands in virtually all circumstances…… Ligand selection is a critical consideration in many practical areas, including bioinorganic and medicinal chemistry, homogeneous catalysis, and environmental chemistry.“37
Therefore, we will need to understand ligands and coordination complexes in more detail to help us get out of the mess that we are in. Please engage yourself in that process as it appears that it will become very important in understanding the human health effects that are in place as we speak.
An introduction to this topic involves what is called the “spectrochemical series”. Fortunately there is a knowledge base available to help us understand what ligands (or chemical structures) are more likely to attach to metal ions, such as iron, than others are. Three fields of study that are helpful in this regard are:38
1. The Spectrochemical Series
2. Ligand Field Theory
3. Crystal Field Theory.
The latter two topics are more advanced fields of chemistry study and can only be briefly mentioned in this report. The latter two subjects, Ligand Field Theory and Crystal Field Theory, help us to understand how the spectrochemical series has developed. In this paper, we need to focus on this end result to start with and to at least become familiar with the spectrochemical series.
The spectrochemical series is a ranking of ligands, according to what are called weak field ligands and strong field ligands. Both abbreviated and longer form tabulations of the spectrochemical series exist depending upon the level of investigation. An example of an abbreviated spectrochemical series is as follows:39
|
||||
Recall that the most important feature of a coordination compound is the donation of a pair of electrons by the ligand (i.e., donor) to form a coordinate covalent bond with the metal. As a first generalization, softer metals generally prefer bonds to weak-field ligands and harder metals (e.g,, iron) are more likely to bond with strong field ligands40. It can also be cited that the cyanide ion and carbon monoxide would be expected to have a rather strong affinity for the ferric (3+) ion41. This type of relationship will be critical in our understanding and future direction of research in relation to the altered blood that has been identified in this report. Separate research from a variety of sources42,43 has also disclosed the following list of candidates ions or molecules to consider as potential ligands to the oxidized iron (+3) atom (this list will overlap with the spectrochemical series):
CO, CN-, NH3, H2O, OH-, SO, NO2 S2- N3- NO2-, Cl-, CH3COO
Please be aware that many of the ligands under review above are toxic or interfere with biological processes. As examples, the cyanide ion, azide ion and carbon monoxide are each respiratory inhibitors to some degree. Although an introduction to a significant problem related to oxygen deficiency (methemoglobinemia) will be discussed later in this report, much research remains to be tackled on the subject of ligands and oxidized iron. Please consider the support of this research if you are so inclined.
9. Spectral Analysis of the Blood and a Comparison to the Growth Spectrum:
Extensive spectrometric analysis human blood is the original basis for this report. It was observed early on in the process that the expected spectrum of normal hemoglobin was not being observed using blood samples from a variety of individuals. This required establishing a “reference spectrum” for hemoglobin based upon that of record and upon historical public data. Please review the previous paper entitled Altered Blood44 for an introduction to the situation at hand. This paper remains current and accurate with the information acquired and analysis completed thus far.
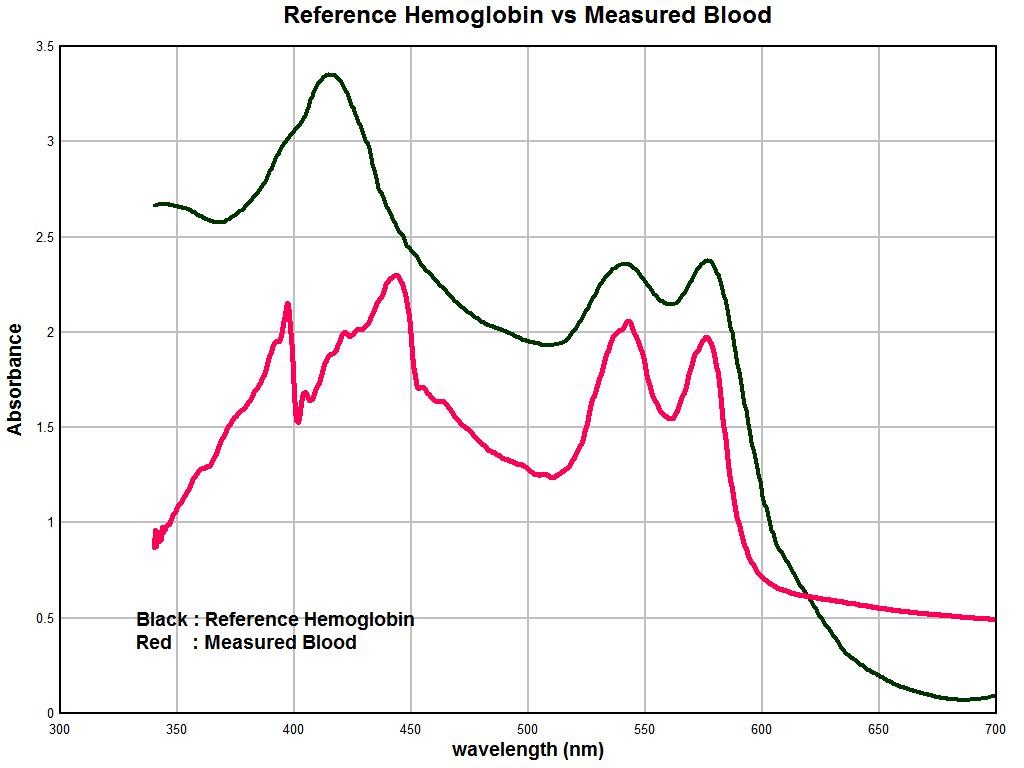
The graphs below show the general nature of the predicament. The purpose of this section will be to summarize only briefly the work of several weeks of observation and investigation of sample hemoglobin vs the reference spectrum that has been established.
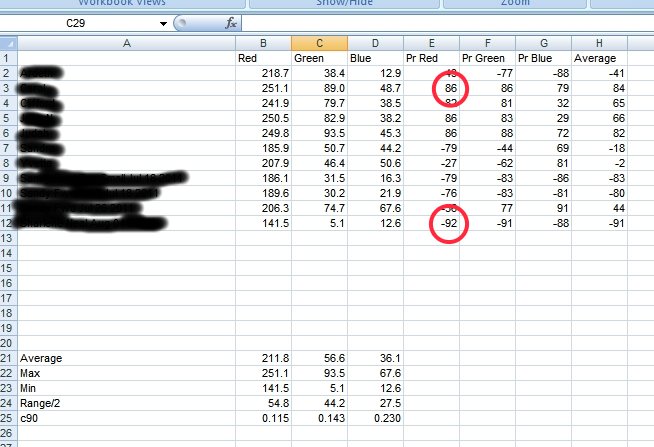
The black line is the reference spectrum for hemoglobin that has been established through examination of the literature and available tabulated data. The red line is the average spectrum of approximately ten individuals over the same visible light wavelength range. Clearly there is a significant difference. A salient change that can be identified is the appearance of two strong peaks in the vicinity at approximately 397 nanometers (nm) and 448 nm. These strong peaks substitute themselves for the prominent expected peak at approximately 414 nm. The magnitude of absorbance can vary strongly according to concentration levels so the magnitude of the peaks so there must be some latitude given to the conclusions related to that aspect. Nevertheless, in general we see that the magnitude of absorbance is strongly reduced in the measured spectrum vs. the reference spectrum, especially in the range of 300-350nm.
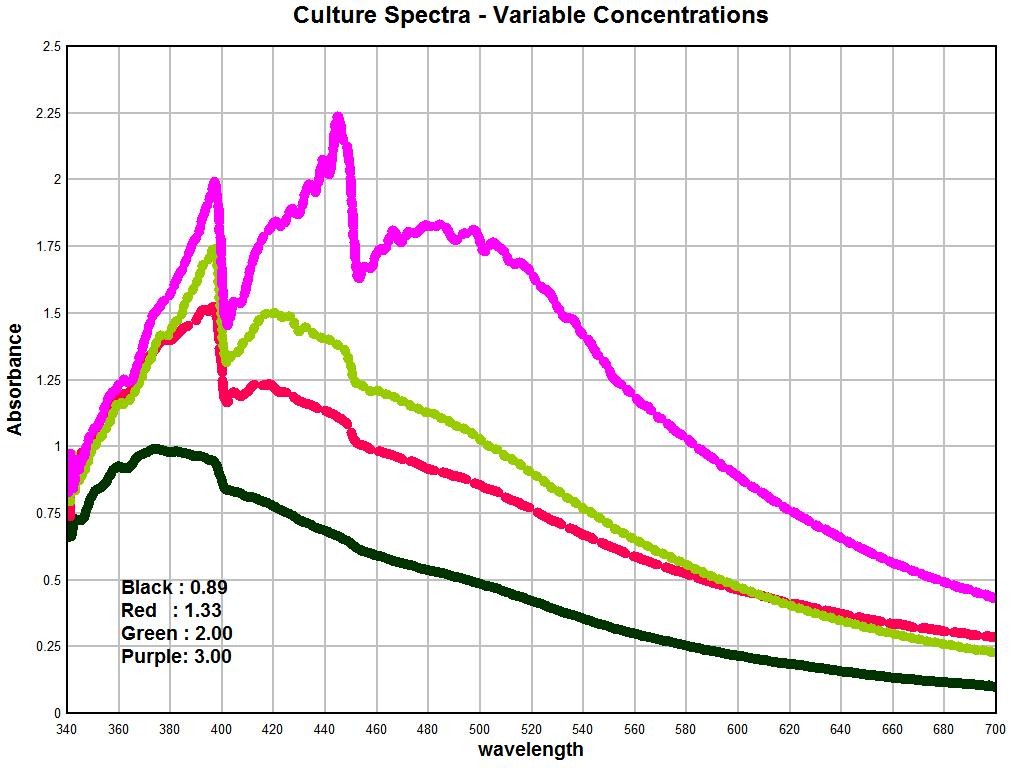
The difficulty then becomes to explain these sharp differences between the spectrums. We can begin this analysis by examining the spectrum of the cultures as they have been developed from oral samples and examples of this work are shown below.
The graph above shows the spectrum of the culture as developed from oral samples. The primary variable within the graph is that of concentration. These graphs show the importance of concentration and how it can affect the geometry of the spectrum. It can be seen in general that an increase in concentration causes a corresponding increase in the absorbance; this is an expected consequence of Beer’s Law is it relates to spectroscopy. It is also of special interest to note that with sufficient concentrations that a second peak appears at approximately 448nm; this peak was simply not observable at low concentration levels. A calibration curve for the concentration of the culture mass has been developed from this work. A fair amount of culture mass is required to produce the highest concentration levels shown; these details of solution preparation can be described further as time progresses. It has already been reported that the solutions are produced primarily with the use of a strong alkalizer (sodium hydroxide) and heat; this method is successful in breaking down the filament nature of the culture to a sufficient degree.
There is an extremely important observation that is to be made from these graphs shown here. It is that the geometry of the peaks of the culture, as it has been developed from oral filament samples, is essentially identical to those deviations that are reported in the measured hemoglobin spectrum shown immediately prior. Within the culture spectrum, we see corresponding strong peaks at approximately 397nm and 448nm, exactly the same peak structure that is apparent in the hemoglobin spectrum under measurement in a sample of individuals. This suggests, in a highly logical and sensible fashion, that we should consider looking at the growth of the organism as a significant factor on the alteration of the hemoglobin spectrum as it is being directly measured.
The next issue of importance is to identify what is the underlying nature of the culture, or organism, spectrum. A spectrum in itself is valuable for its uniqueness, but the interpretation of the underlying spectrum is a much more involved affair. It involves a body of knowledge than can represent a profession it is own right. Some of the factors that affect the manifestation of the spectrum include the elements involved, the types of molecular bonds involved and the energy states of those atoms or molecules. I do not profess to know that science to that level of detail to immediately be able to interpret a visual light spectrum at the elemental and atomic bond level; by the same token the subject matter is not entirely foreign to me at this stage of study.
The process of investigation on my end is too laborious and time consuming to describe here, and the extensive time and effort extended is to be summarized in a succinct manner for your benefit In that protracted process, the spectrum of iron salts has also been examined in some detail. Suffice it to say that the spectrum of the ferric ion (3+) in solution matches remarkably well with the spectra culture and oral sample spectrums, especially in the range of 300 – 475nm where the deviations reported above most strongly occur. This was indeed the discovery that has motivated the intensive focus on iron with respect to this particular growth form, or “organism”, as it were. It is also the very reason why the qualitative chemical studies described above were developed. I have attempted to approach the problem from numerous angles to seek a consistent resolution to the problem. At this point, it seems fair to claim that such a consistent resolution has been reached. The role of iron in the oxidized state (3+) and its importance in the growth of the organism, from this researcher’s perspective, appears to be positively established.
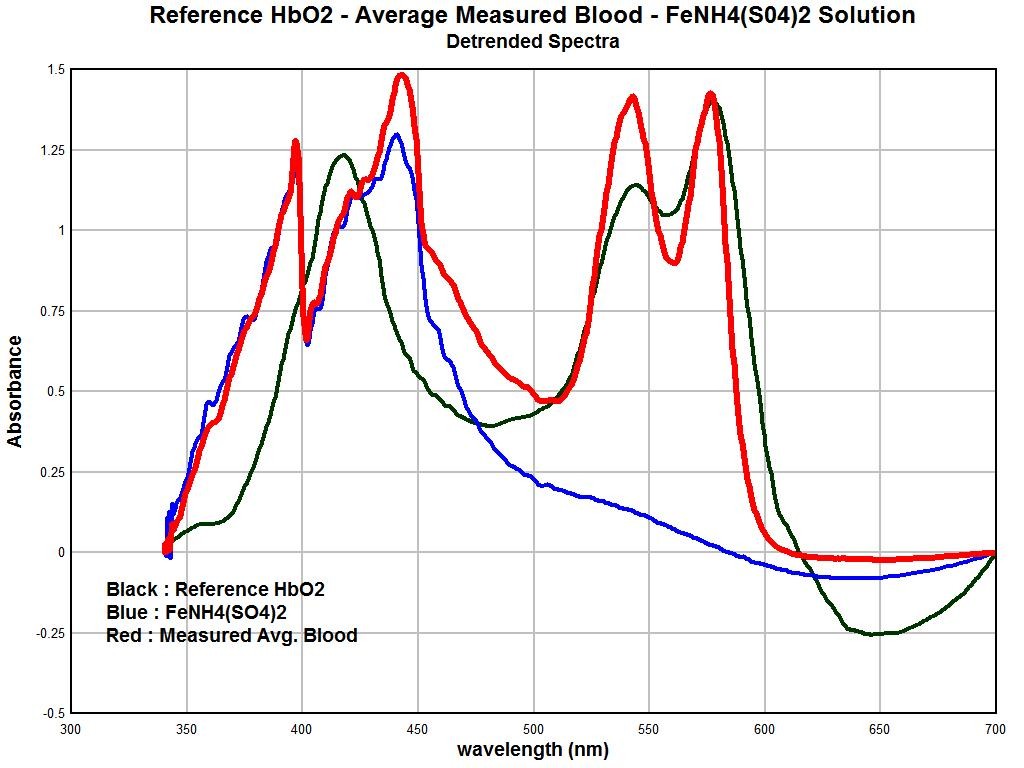
The final graph in this section shows the degree of overlap that is occurring between the hemoglobin spectrum as it is being measured, the spectrum of the oral and culture samples, and the spectrum of the ferric ion (3+) in solution. The degree of similarity and overlap is actually quite remarkable and further solidifies the arguments that are presented within this paper.
In these graphs, the trends of each individual spectrum has been removed. This has the advantage of essentially normalizing the magnitudes of the graph so that we can focus on the degree of similarity of the absorption peaks. We have three different spectra shown here. The red line is the average measured spectrum of hemoglobin from a sample of approximately ten individuals. The black line is the spectrum of the “reference hemoglobin” as it has been obtained from the available public sources. The blue line is the spectrum of a dissolved ferric (3+) salt, specifically iron ammonium sulfate. There are some important observations to me made here that reiterate the degree of similarity that has been established prior. We see a very close match between the spectrums of the measured hemoglobin spectrum and the ferric ion (3+) in the lower half of the visible spectrum (350 – 475nm). This strongly suggests that the ferric (3+) form of’ iron is intimately involved in the deviation of the measured hemoglobin spectrum from the reference hemoglobin spectrum It is indeed the basis of this thesis, as the body of evidence established now demonstrates that this is exactly the case.
Secondly, we see that the magnitude of the spectrum of the ferric ion drops off radically in the upper half of the spectrum, i.e., 475 -700nm. This means that we would expect the ferric ion to have much less influence upon the spectrum of hemoglobin within that range. This is also exactly what we find. We notice that the reference hemoglobin spectrum and the measured hemoglobin spectrum actually compare reasonably well in the upper half of the visible light spectrum. This spectral analysis establishes the case quite strongly, therefore, that the ferric (3+) ion form plays a prominent role in the alteration of blood as it has been measured from several individuals. It is at this point that we must recall that deviation of the iron in the blood from the normal state of Fe(2+) to that of Fe(3+) presents serious health consequences. The most important of these is the inability of iron in the ferric state within blood to bind to oxygen. This leads us to the next topic below.
10. Methemoglobinemia and Hypoxia:
Now that certain results have been established, we must anticipate and begin to deal with the consequences of those results, should they be proven to be true. To reiterate, these results present themselves in two primary forms:
1. The evidence indicates that the growth form central to the Morgellons condition utilizes iron in a ferric (3+) state for its own growth, development and sustenance.
2. The evidence indicates that human blood is altered significantly as a result of the presence of the organism within the blood. This alteration encompasses a partial change of the oxidation state of the iron within the hemoglobin from a ferrous (2+) to a ferric (3+) state. Iron in the ferric state (3+) within hemoglobin is unable to bind to oxygen.
If these findings are true, we are required to pursue the next logical line of investigation, i.e, diminished oxygen carrying capacity of the blood. There is a known medical condition for this change within the blood, and it is called methemoglobinemia. Methemoglobinemia is the transformation of normal hemoglobin (oxyhemoglobin) to a deoxygentated state. Methoglobinemia is caused by the oxidation of the ferrous ion (2+) to the ferric state (3+). Ferric iron is chemically useless for respiration45. Methemoglobinema can exist at varying levels, and is usually expressed as a percentage of the total hemoglobin of the blood. It is a normal state to have approximately one to two percent of methemoglobinemia (ferric ion) in the blood46.
Mild methemoglobinemia, on the order of 2 – 10%, is generally well tolerated by individuals and usual presents no obvious or apparent symptoms47. There is, nevertheless, a diminished capacity of the blood to carry oxygen at this stage and the effects are not to be dismissed as we shall discuss further. At levels from 10 -15%, cyanosis will occur with the skin taking on a blue/gray cast or appearance. Higher levels still, e.g, above 20% can cause dizziness, increased heart rate and anxiety. Levels greater that 50% are associated with breathlessness, fatigue, confusion, drowsiness. Comas, seizures may also occur at this level. Methemoglobinemia at 70% or greater is usually fatal48.
From the results of this paper, it the following hypothesis can be presented. It it is accepted that the Morgellons growth form is responsible for a partial alteration of the blood from a ferrous to a ferric state, it follows that those with a more serious manifestation of the condition may demonstrate a tendency toward increased levels of methemoglobinemia. Whether or not this is the case is to be determined by the medical profession at some time and place, however, initial investigative work on this proposal will be presented within this report. Although only a preliminary and tentative analysis, one spectrometric/chemical analysis made has indicated a potential level of an approximate 7% oxidation state (3+) in the average hemoglobin measurement of this report. This level would be without obvious visible symptoms as described earlier. This analysis requires further examination to substantiate that finding.
Obviously there are many purported and claimed manifestations and variations of the so-called “Morgellons” condition, and this paper is not able to encompass that scope or debate. The work of this researcher places a focus on what is perceived to be an originating growth form as identified through several years of observation and analysis of various sample types (primarily filamentous in nature.) This paper will simply not have the capacity to discuss all of the ramifications of diminished oxygen capacity of the blood; it will have to suffice at this point to state that this process of discovery must now begin. Some occasional comments on the subject will be presented as time and circumstance allow me. Degrees of hypoxia and its effect upon cellular metabolism will also become a point of investigation in our future. As a starter, please recall an opening statement that all energy to the body is dependent upon respiration.
Finally, to end this section for the time being, a visual representation of the nature of methemoglobinemia (deoxyhemoglobin) is repeated below for the reader’s reference.
The dexoxygenated heme molecule (model) shown with oxygen atoms removed (red) (left)
The oxygenated heme molecule(model) shown with oxygen atoms attached. (right)
Source : Protein Data Bank
http://pdb101.rcsb.org/learn/resource/oxygen-binding-in-hemoglobin-gif
11. Ionization and Bond Disassociation Energy : The Cost of Oxidation:
It requires energy to form molecules49. It requires energy to remove an electron, i.e., oxidize an element or molecule49. And it takes energy to break bonds50. What this means, in simple terms, is that the theft of energy from our cells to serve the metabolic requirements of a pathological organism comes at a price to our body and our health. The removal of an electron is called the ionization energy. These are referred to as the first ionization energy, second ionization energy, third ionization energy, etc. corresponding to the removal of one, two and three electrons respectively.. There is energy required to remove two electrons from iron in the elemental state to the oxidation state of iron (Fe2+). This oxidation state is the one that is most commonly found in nature. To remove an additional electron, and bring iron to the Fe(3+) state requires even more energy. Oxidation essentially represents the stealing of electrons from one element or molecule by another.
The first ionization energy for iron is 7.9 electron volts (eV) (~760 kilojoules (kJ) per mole), the second ionization energy is 16.2 eV (1560 kJ per mole) and the third ionization energy is 30.6 ev (2960 kJ per mole)51. What this shows us is that it takes almost twice as much energy to remove the electron to change the iron from the ferrous (Fe2+) state to the ferric (Fe3+) state as it did to remove two electrons to change it from the elemental form to the Fe(2+) state. From an energy standpoint, therefore, the oxidation of iron referred to in this paper requires a relatively strong energy investment.
To get some sense of what this energy level actually means, let us translate what is happening in the blood to something more tangible for us to visualize. If we assume a 5% reduction in oxygenated hemoglobin over a three month period (the approximate life cycle of red blood cells), this will translate to an energy requirement of approximately 3240 joules over this three month period.
[Humans have roughly 2.5E13 red blood cells; 280E6 molecules of hemoglobin in each red bllood cell; 7E21 molecules of hemoglobin in each red blood cell; four heme molecules per red blood cell; approx. 2.8E22 Fe2+ iron atoms in the human body; at 5% oxidation 1.4E21 atoms in the Fe(3+) state ; .0023 moles of iron in the Fe(3+) state, .0023(2960kJ/M – 1560kJ/M) = approx. 3260 joules over a three month period.]
It takes approximately one joule of energy to raise an apple over your head. If these approximate calculations are correct, this would be equal to raising roughly 3000+ apples over your head in a three month period. This equates to roughly three dozen presses per day; this is not exactly trivial since this energy expended should be serving your own interests vs. the metabolism of a detrimental pathogen. Regardless of the computations, the energy is stolen energy.
It also takes energy to break chemical bonds. In this case, we can at least look at the separation between the iron and oxygen atoms. The bond dissociation energy for the iron-oxygen bond is 409 kJ per mole52. Again, even though we are making some approximations, this leads to roughly another 940 joules of energy released in a damaging manner if we assume the same three month period. Add lifting another 1000 apples to your detriment.
And lastly, it takes energy to form molecules. This brings up the entire discussion of ligands again, as new molecules will form with the oxidized iron, many of them harmful to the human body. For example, the ferricyanide complexes is one of the most likely complexes to form from the altered iron, and it is toxic as well. To form that complex, or other complexes that result from the spectrochemical series, will require additional energy. From an energy standpoint alone, you are doing bench presses on a regular basis and your health is suffering in the process.
There is a cost for the oxidation of the iron in our bodies, and that cost is to one’s health.
12. Bacterial Requirements for Iron in the Blood:
For those patient enough to follow the course of this paper, it is fair to state that significant efforts have been expended, from both a laboratory and a research point of view, to demonstrate that changes in iron and the utilization of iron in a pathogenic sense are at the heart of the Morgellons issue, at least from the perspective of this researcher. The changes and impact upon the body have been demonstrated and they will continue to be so. For those that are inclined to accept conclusions more readily from the conventional literature, the following is provided from the section entitled, Chemistry and Life, The Battle for Iron in Living Systems53:
“A bacterium that infects the blood requires a source of iron if it is to grow and reproduce.”
Recognition of the truth and simplicity of this statement may have saved a great deal of time and effort, but this particular reference was not found until the same conclusion was reached from direct experience. The time and effort has not been lost by any means, as there is now a deeper understanding from whence this statement comes. Let us now add some complimentary information to the direct knowledge given to us from the statement above. First of all, it is true that the work does not positively identify the sub-micron spherical originating organism as a known or specific bacterium. It does, however, seem to be a most relevant consideration. At this point, it is best to refer the reader to a prior paper that expresses the proposition of essentially an “engineered” organism54. that combines the prokaryote, eukaryote and archaea life forms. The bacterial form is a subset of this larger life classification system and the above statement holds as true and relevant to the work. On a more general level, we can delve into the question further and ask whether bacterial forms are commonly involved in the consumption of iron. The answer is yes. From a variety of sources, we can only confirm further the findings of the current research; the fact that bacterial forms require iron for their survival is readily verifiable:
“Like their human hosts, bacteria need iron to survive and they must obtain that iron from the environment. While humans obtain iron primarily through the food they eat, bacteria have evolved complex and diverse mechanisms to allow them access to iron… Iron is the single most important micronutrient bacteria need to survive… understanding how these bacteria survived within us is a critical element of learning how to defeat them55.”
“Bacteria metabolize iron as a food source and release iron oxide as a waste product…bacterial waste lowers pH56.”
“The term iron bacteria does not refer to a specific genus or species but rather to those bacteria in which reduced iron plays an important role in their metabolism… A great variety of bacteria can be involved in this process. The “true” iron bacteria are those in which the oxidation of iron is an important source for their metabolic energy. This group is most often associated with filamentous or stalked forms…57“
“Bacterial requirements for growth include sources of energy, “organic” carbon (e.g., sugars and fatty acids) and metal ions (e.g., iron)…..Nutrient Requirements: These include sources of organic carbon, nitrogen, phosphorus, sulfur and metal ions including iron. Bacteria secrete small molecules that bind iron (siderophores). Siderophores (with bound iron) are then internalized via receptors by the bacterial cell58.”
“Siderophores are biosynthetically produced and secreted by many bacteria, yeasts, fungi and plants, to scavenge for ferric ion (Fe3+). They are selective iron-chelators that have an extremely high affinity for binding this trivalent metal ion….. The emerging overall picture is that ion metabolism plays an extremely important role during bacterial infections.59.”
“The ability of pathogens to obtain iron from transferrins, ferritin, hemoglobin, and other iron-containing proteins of their host is central to whether they live or die..Some invading bacteria respond by producing specific iron chelators – siderophores – that remove the iron from the host sources60.”
| “Iron is one of the most common elements in the Earth’s crust and forms a ready oxidation state. Bacteria use this as a source of energy and as a means of waste disposal.. Iron metabolism is also a significant part of bacterial virulence…It has been established experimentally by injecting iron soluble compounds into test animals with infections that adding more iron causes the bacteria to thrive….Bacteria put out compounds, called siderophores, which attract and bond free iron compounds by chemical processes; these are then oxidized and excreted as a byproduct61.” “Iron (Fe) has long been a recognized physiological requirement for life, yet for many organisms… its role extends well beyond that of a nutritional necessity. Fe(II) can function as an electron source for iron-oxidizing microorganisms under both oxic and anoxic conditions and Fe(III) can function as a terminal acceptor under anoxic conditions for iron-reducing organisms62.” “Given the role of free iron in creating DNA damage, it is unsurprising that bacteria have evolved methods to scavenge it….Despite the sophisticated biochemical and genetic strategies that can be brought to bear upon bacteria, we still know remarkably little about the physical mechanisms of iron transport, storage, and regulation, and virtually nothing about iron trafficking and its insertion into metalloproteins. These areas are ripe for future work63.” |
As a parting comment within this section, there is a class of siderophores produced by certain bacteria that bind in particular to iron in the Fe(3+) state64,65,66. These siderophores are called enterbactin. What distinguishes this class is an incredibly strong bond to the iron (i.e., chelation) in the 3+ state, and it can not be broken through normal physiological processes or with such proteins as transferrin. This type of siderophore is usually found in Gram-negative forms of bacteria. Readers may recall that several years ago gram stain tests were repeatedly performed on the bacterial-like organism under study and discussion here. The results of those tests were Gram-negative. Enterobactin and ferrichrome therefore emerge as important targets of further research within the iron dilemma.
The journey to the current state of knowledge has been a long one, and for that matter, it has been unnecessarily long. We can, nevertheless, take some solace in knowing that some findings of importance are before us. There is also now a stronger sense of direction of what is required and what is to be done. If you would like to hasten this process, you have the opportunity to do so67.
13. The Oral Filament and Red Wine Reaction Resolved
It has long been a mystery as to why there is such a definite and visible reaction, especially of color, between the oral filament samples and red wine or related solutions. This mystery has now been resolved with a combination of investigative chemical research and the knowledge of iron changes in the body. The reason for the strong reaction is the formation of a metal complex of Fe(3+) in combination with the pigments found in red wine. Once again, at least some knowledge of coordination chemistry in combination with transition metal characteristics proves fruitful. Grapes, red wine and many related fruits or vegetables contain a group of pigments called anthocyanins. A search of the literature will reveal that iron, especially in the ferric state (Fe3+), will form metal complexes with these pigments68,69,70,71,72. The color of many of these metal complexes is often a deep purple, exactly that which is known to occur in the combination of the oral filaments with the red wine.
It is also of interest to learn that the molecular structure of the complex, i.e, the combination of Fe(3+) with anthocyanins, has a chemical structure with some similarity to that of ferrichromes. Ferrichromes are a product of bacterial consumption of iron, and they involve the formation of strong chemical bonds that tie up the iron within a ferric metal complex.
It is the understanding of the chemistry of iron in its various states along with the important but more complex branch of coordination chemistry that has allowed us to understand the nature of the ferric iron – red wine reaction. This understanding provides one further level of verification and confirmation of the change of iron that occurs within the body as a direct result of the pathogenic metabolism.
14. Some Health Implications; The Value of the Holistic Approach to Medicine
For those that seek a pill to remedy the dilemmas of the Morgellons “situation”, you must seek elsewhere. My work will not offer such a simple path for you. The research of the past several years on the bio-engineering issues has been a journey of education in health, myself included. Out of this research I have developed a level of respect for the wholistic approach to medicine and for those who practice it well. Those who have this knowledge coupled with strong foundations of chemistry, biology and physics will earn even greater respect as they are likely to be our better sources for counsel.
Let us start with some of the controversy in language regarding the issue of a “condition” vs. a “disease”. As the work indicates that the general population is subject to the pathogenic forms under study, it becomes even a more sensitive issue as we confront our own involvement irrespective of our wishes or personal belief systems. I will start this discussion with reference to a rather hefty tome, Robbins Pathologic Basis of Disease73. This book may not be bedside reading for most of us, but in many ways it should be. It is a real eye opener for the uninitiated. For now, let us introduce just a few insights that this reference will provide to us. First, what pathology actually refers to, in the origin of the word, is suffering. We can play with semantics all that we wish, but those suffer in a biological sense will need to deal with the reality of the terms pathogen and disease. Cells, tissues and organs that sustain injury are at the root of the study of pathology. Study the textbook and reach your own conclusions as to the severity of affliction. It is a diminishment to the reality and seriousness of the issue if we classify the current situation as a “condition” for our own personal palatabilities and psychological comfort. It is difficult to deny the classification of “Morgellons” as a disease or as of pathogenic form if you look at the underlying mechanisms of damage that have taken and are taking place. I may not please the reader but that is not my purpose here; it is to confront and comprehend the reality of our existence be it kind or brutal.
The next topic concerns what we must read to get started with our education on pathology. Robbins’ book is roughly 1500 pages long. If we can digest even a portion of the first 40 pages, we have done ourselves a great service. It will be found that this introductory section alone will spell out the majority of the specific mechanisms and actions of injury to our health at the cellular level; this foundation will underlie the remainder of the book which will go on to address injury to further organs. A knowledge of cellular injury is crucial to our understanding of any disease and how it works its damage upon the body. It is not especially relevant at this stage of our discussion to single out the particular malady at hand; understand the mechanisms of cellular damage in general and tremendous progress can be made in the path to better health and health understanding. This particular book is more than 20 years old and yet the level of knowledge on how disease damages the body is evident, open and obvious to those that are willing to take a look at it. This knowledge can be applied to any circumstance of illness that I can foresee, past or present, including our current problems. It will be to our benefit to invest this effort for what awaits us as we learn to apply that knowledge. A standard and comprehensive book on pathology is at the very heart of medical knowledge and application; those with a wholistic approach to medicine that seek the source of a problem versus a prescribed band-aid deserve our greatest respect and honor. This particular chapter of this paper will never be completed as the pathways and connections within the body never cease to amaze me. I am an infant in these wonders myself and must admit my own negligence with respect to the understandings of physiology, disease and health. In many ways, the course to better health has been spelled out for us many years, even decades, ago and it is our job to at least acquaint ourselves with the work that has already been done for us.
This preparation established, let us at least briefly mention what the four systems of damage (i.e., vulnerabilities) are to the cells in our bodies through disease74: These criteria form the very basis of pathology:
1. Damage to the cell wall or membrane.
2. Aerobic respiration (i.e., oxygen based respiration) and the production of energy within the cells.
3. The creation of enzymes and proteins within the cells.
4. Preservation of the genetic integrity of the cells.
My work indicates, at this point, that every one of these critical factors underlying damage to our bodies is underway or is likely to be underway within the mechanisms of the Morgellons pathogenic forms. It is much harder to prove that any one of them is not involved than it is to make the case that they are in effect. If this is to be accepted, the very core, foundation and definition of “disease” is in full bloom here and it is only a diversion to avoid that unpleasant reality. The necessary job is to understand the forces at work in great detail from a biochemical perspective and then get to work on the solutions to the problems that they pose for us a species and as a whole. The stakes are serious enough; make your decision as to when and how your are going to become involved in your own survival and those that follow.
Let us give some introductory examples or thoughts as to how and why these factors are likely to be involved.
In terms of damage to the cell wall or membrane, the damage to the red blood cell walls has been aptly documented. Please see the previous paper entitled “A Mechanism of Blood Damage75“.
In terms of aerobic respiration, it is also now clear that the oxygen carrying capacity of the blood is expected to decreaseThe prospect of this finding was first recorded in March of this year. Sufficient opportunity has been afforded to return to laboratory studies during the past few weeks and the original findings have been confirmed at a higher level. In the interim, a greater understanding of the likely molecular structure and bonding arrangement of the proteinaceous complex has been deduced, or at least hopefully this is the case. Sufficient resources, had they become available at an earlier time, would have rapidly advanced the painstaking studies that have brought us to the current state of knowledge. This state of knowledge remains in the majority, highly unfinished, but it is believed that an important level of progress has likely been achieved under the current work. as a result of the increased oxidation level (Fe3+) of iron with the blood. Recall that oxygen does not bind to hemoglobin when the iron is in the Fe (3+) state. If the oxygen carrying capacity of the blood is diminished, the production of energy (ATP) is also expected to be diminished.
With regard to enzyme (most enzymes are proteins) and protein production within the cells, it is a fact that essentially all cellular reactions that take place within the body require enzymes for those reactions to occur76. And, as an example of the tie between iron and enzymes, approximately one-third of all enzymes require metal ions77 and iron is also an essential component of many proteins and enzymes78. If cellular metabolism is interfered with (i.e, the production of energy by the mitochondria) then the catalytic reactions involving enzymes within the cells are disrupted.
Lastly, oxidation in the body produces free radicals79,80; an excess of oxidation can exacerbate this issue. Free radicals can damage DNA and can result in the alteration of a given gene81. Iron is involved in the production of DNA82. The alteration of the iron state of the blood can therefore also jeopardize the genetic integrity of the cell.
What we see, therefore is that any alteration or interference of iron metabolism in the body leads to serous and systemic degradation in human health and functioning. In addition, the very mechanisms of damage (as defined from a pathological perspective) to the cells are identified as being factors of the Morgellons situation and they fully satisfy the definition of a diseased organism. It is this comprehensive and systemic effect upon the body which necessitates the call to integrative and wholistic medicine with a strong foundation in biochemistry. It is not anticipated at this point that a myopic perspective on either symptoms or effects is likely to be beneficial at the level that is required to establish health.
15. Identification of physiological conditions that are in probable conjunction with the condition:
Based upon the understanding that has been presented thus far, there exists a set of physiological conditions that is expected to be more likely to occur in the “Morgellons affected individual” than in the general population. It is a probabilistic offering only. This information is not intended to be diagnostic in any sense and the postulates are presented solely as a result of analytic and observational research. The information is offered to the medical community for their evaluation and assessment as the issue is approached with greater seriousness in the future. There is no guarantee or implied guarantee that any of the following symptoms or conditions will occur; only that they may deserve consideration by the medical community as the situation is researched further. The list of candidate effects upon the body may or may not include:
1. An increased level of acidity in the body (may be most easily assessed by urine pH testing).
2. Diminished oxygen carrying capacity of the blood.
3. Lower energy levels due to interference in the ATP production cycle; greater fatigue.
4. The presence of filament structures (ferric iron – anthocyanin complexes) within oral samples.
5. Recent research indicates that the urinary tract may be equally affected with the presence of the filament structures.
6. The presence of a bacterial-like component (chlamydia-like) within or surrounding the red blood cells.
7. Chronic decreased body temperature.
8. Respiratory problems, including proclivities toward a chronic cough or walking pneumonia-like symptoms.
9. Skin manifestation at the more developed levels (the skin is an excretory organ).
10. The impact of increased oxidation, greater free radical presence and their damaging effects upon the body.
11. Tooth decay or loss.
12. The smoking population may exhibit an increased incidence of the condition due to additional oxygen inhibition within the blood.
13. Liver toxicity, gall bladder and bile duct complications.
14. Potential reduction in arterial transport; increased blood pressure.
15. Potential proclivity toward increased cancer incidence due to an expected increase in aneroboic metabolism.
16. Additional unidentified systemic damage in conjunction with the pathological mechanisms of cell injury identified.
16. A Proposed Spectral Analysis Project:
A relatively simple method to assess whether or not the oxygen content of the blood is abnormally low has been created. The method uses the combination of an ordinary computer scanner along with statistical analysis. Before this method is outlined further, I would like to give due credit to Fathima Shihana, BSc with the authored paper entitled “A Simple Quantitative Bedside Test to Determine Methemoglobin” from the Annals of Emergence Medicine83. This paper has served as the inspiration for the approach described here.
A spectrometer is a relatively costly instrument and it availability is limited. The paper above describes a method whereby an ordinary scanner can be used to establish a calibrated relationship between the color of blood (as recorded by a color scanner) and the loss of oxygen content (methoglobinemia) within that same blood. The cleverness of the idea resides in the fact that a color scanner, along with suitable analysis software, is essentially a spectrometer in its own right. Any color combination may be broken down into quantitative measurements of the red, blue and green channels of that color, and a scanner ingeniously serves as a readily acceptable spectrometer in its own right.
The paper referred to deals with situations of methoglobinemia that be lethal or extremely injurious to life; the project here is operating on a much more subtle level in an effort to determine both lesser magnitudes of the condition (i.e., asymptomatic) and finer gradations within. Without the advantage of calibration against known lab standards, the scanner still serves as an excellent and simple tool for relative changes in the condition of the blood. Highly oxygenated blood is a rich red in color. Oxygen deprived blood is more bluish and color and blood devoid of oxygen is brown. Our goal with the current project is to be able to determine relatively minor (but nevertheless significant) shifts in the color of blood from red toward the blue portion of the spectrum.
As shown below, a modern computer color scanner can be used as a three channel (red, green, blue) spectrometer and it can be used to establish a highly unique signature for an appropriate sample. The proposed project of blood spectral analysis processes the sample data in a unique fashion, but the spirit of the research paper referred to above remains the foundation of the approach.
x.
In practice, it has been found that the red channel is sufficient to identify color shifts within the blood that indicate a decreased oxygen supply to the blood; If you would like to participate in this research project, please send correspondence to info@carnicominstitute.org and the particulars can be described. The only essential requirement to participate in the project is that of a color scanner. Please be aware that no individual feedback or assessment will be provided to those that participate in this project; any data will be handled in a statistical sense and any data analysis will be presented to the public in an anonymous fashion. If the medical community becomes involved in this research the prospects of discussion may be able to widen.
What follows below is an example of the processing that the project entails, including the scan of a drop of blood by two separate individuals and the statistical processing of a group of individuals that have contributed to the research project:
Individual A.
A scan of the blood of the individual. This individual has no outward manifestations of the “Morgellons” symptoms. The red channel of the spectrum has been analyzed from a statistical perspective. The individual has a relative rank in the +86% (-100% to +100%) percentile, indicating a shift of the color toward the red portion of the spectrum. This dominance of the red portion of the spectrum indicates more highly oxygenated blood within the group sample.
Individual B.
A scan of the blood of the individual. This individual has stated and demonstrated significant skin manifestations of the “Morgellons” symptoms. The red channel of the spectrum has been analyzed from a statistical perspective. The individual has a relative rank in the -92% percentile (-100% to 100%), indicating a shift of the color toward the blue portion of the spectrum. This shift towards the blue portion of the spectrum indicates a decrease in the oxygen level of the blood of the individual. This finding is in accordance with the primary thesis of this paper.
The spreadsheet analysis for the sample group. The worksheet analyzes the statistical properties of the sample group (11 individuals) with respect to the average spectrums of the red, green and blue channels. In practice, it is found that the red channel shifts in the spectrum are sufficient to characterize the deviations in color. The color changes, or shifts, are an expression of the oxygen content of the blood. The sample group at this time is limited and also has a high probability of being polarized with a limited data set. A broader sample group is expected to reveal a more even distribution of oxygen supply and deficiency. If you would like to contribute to this research project, please contact info@carnicominstitute.org for the particulars. No individual data will be provided to participants; all analysis and presentation will be from an anonymous statistical perspective.
.
17. A Review of Proposed Mitigation Strategies:
With the understanding of how a malady affects the body, we are in a stronger position to develop strategies to mitigate the damage. The better approach is to put a stop to the problem, but that requires a broader coordination and alliance than has been achieved thus far. We can at least consider and establish some defenses while the forces of political and social organization continue to arm themselves. What follows here are merely suggestions to consider; they are in no way to interpreted as therapeutic or diagnostic in approach. Each of the following strategies has been developed as a direct response to laboratory conditions or academic study; they are not formulated within any formal medical framework. Each individual is responsible for consulting with the medical professionals of their choice and the following information is provided solely for consideration within that consultation. Many of these items have been mentioned previously and the list has accumulated in a gradual fashion. Understanding the extent of the problem, it is not intended that the “list” is complete; in fact it would seem that it is only a beginning. It will be noticed that many of these strategies can apply to human health in general. The primary mechanisms of many diseases are actually few in number and these have been enumerated in the discussion of pathology above.
All being said, let us proceed with some strategies for mitigation of the “condition“.
1. Alkalization of the body would appear to be a beneficial practice in general with respect to disease84,85,86. It has been identified that the organism flourishes within an acidic environment87,88. It is also known that biochemical processes usually take place within a specific pH range, including the growth of pathogenic forms89,90,91.
2. The research indicates that excessive oxidation is detrimental to health. This topic has also been discussed previously in an earlier paper92. Common oxidizers include the bleaches, peroxides and ozone. The research indicates, from the vantage point of this researcher, that internal use of these substances is likely to be harmful to human health. We do not solve the problem of oxidation within the body by necessarily increasing the intake of oxygen. Indeed, one of primary arguments of this paper is that the blood of the affected individual has been oxidized in a fashion that has the net effect of decreasing the oxygen carrying capacity of the blood. Excessive and misplaced oxidation also creates free radicals, which as been noted, “wreak havoc in the living system.”We do not solve that problem by taking more oxygen; we work on the problem by hindering the oxidative process. The manner in which this process is conducted in the chemical world is known as reduction. In common terms, the appropriate term is that of an anti-oxidant, and many of us are familiar with that parlance.
I take stock in the following statement, again from Coltrane93:
“Once free radicals are formed, how does the body get rid of them? There are several systems that contribute to termination or inactivation of free radical reactions:
1. ….Antioxidants (.e.g, vitamins, glutathion, transferrin..)
2. Enzymes.”
The statements here are direct and understandable and come from a standard textbook in pathology. It is relatively straightforward that if a problem of excessive oxidation exists within the body, one should strongly consider the role that anti-oxidants play in reversing those effects. It is equally inadvisable, from this researcher’s point of view, to compound the issue with the addition of known strong oxidizers internal to the body
Vitamins, across the board (A, B, C, D,E) are powerful antioxidants. An additional powerful antioxidant identified in the research is that of glutathion. The role of Vitamin C (ascorbic acid) in the inhibition of the culture growth has already been described. There remain many additional anti-oxidants of importance in human health94.
3. Increasing the utilization and absorption of existing iron within the body. Iron is certainly one of the most important elements of the body. Referring to the Linus Pauling Institute95,
“Iron has the longest and best described history among all the micronutrients. It is a key element in the metabolism of almost all living organisms. In humans, iron is an essential component of hundreds of proteins and enzymes.”
One of the findings from the study of coordination chemistry described above is that iron has the ability to bond with numerous other molecules. For example, iron (in the Fe2+ state) preferentially bonds to oxygen. If the iron is altered to the Fe(3+) state. it will no longer bond to oxygen. In this modified state, the iron will then form additional bonds to other molecules, many of which are harmful as has also been described above. The idea of a chelator is to keep the oxygen bound in a protected state where it can not bind so easily with other, often harmful, molecules. Heme itself, within hemoglobin, is a classic example of a chelator. If our iron has been altered to where it becomes free or bound to other molecules (potentially harmful ligands), the solution to that problem would not seem to be to take more iron, any more than increasing the oxygen intake is expected to resolve a problem of oxidation.
The more effective solution would appear to be to keep the iron in a chelated state, where it is bound and protected by the expected molecules and proteins such as heme in the body. This therefore suggests that increased attention would be devoted to the study and role of chelators in human health. It does not seem reasonable that we would automatically pursue a path of increasing iron intake; indeed this process can be quite harmful and dangerous to human health. Again, the importance of consultation with the medical professionals of choice is unequivocally stated; the stakes of the issues we are speaking of are of the highest importance.
4. The inhibition of the growth of iron-consuming bacteria (and bacteria-archea like) forms.
We know now that the organism uses iron for its existence and growth. It appears that iron in the further oxidized state (i.e, Fe3+) is of primary benefit to the organism. We also know, in retrospect, that iron is a critical metabolic element within many of the bacteria (or bacteria-archaea like forms). One strategy that develops with such organism is that of inhibiting the ability of the organism to access or metabolize the iron. This once again brings up the idea of a chelator. This topic has also been discussed in an earlier paper, and introduced the role of human breast milk and its resistance to bacterial forms in infant growth96. Lactoferrin (found in whey) was identified as a potential strong chelating protein within that research. Transferrin is another protein chelator within the human digestive tract that serves a similar purpose, i.e., binding of the iron and consequently it becomes less accessible to iron-consuming bacteria (or bacteria-archea like forms).
5. Improving the flow of bile in the system to further alkalize the body and aid the digestive system. The liver, the gall bladder and the bile duct play an extremely important role in alkalizing the digestive tract. For those that demonstrate a persistent acidic condition within the body it may be beneficial to learn of the importance of bile production and its alkalizing function. An excellent introduction to the physiology of this important aspect of human health may be found at the following site:
Video Series: Liver, Gall Bladder and Bile Duct Physiology
(www.balancedhealthtoday.com/glytamins.html)
An acidic condition can easily be created with a blockage of the bile duct, as the bile is the alkalizing agent within the intestine. Gall bladder removal and gall stones appear to be a frequent occurrence; this would suggest that overloads of toxicity to the liver could well be at the root of this problem. Non-invasive methods of breaking down gall stones (conglomeration of bile) are available to consider, such as Chanca Piedra (breakstone). If the bile flow is restricted, an acidic condition within the body is expected to exist. Knowledge of the physiology of the liver, gall bladder, bile duct and its relationship to digestion may be beneficial in mitigating the consequences of acidity within the body and digestive system.
6. Detoxification of the liver (toxin removal and the breakdown of lipids(fats)). One of the many functions of the liver is to break down fats with the use of bile. If the bile is not being produced or flowing within the digestive system, the fats will accumulate within the liver. The liver also removes toxins from the body. If the liver is not functioning correctly (e.g, from an accumulation of fats or the lack of bile flow) serious consequences to health will ensue.
As an aside and as an unreported event, I received information indirectly many years ago from a U.S. Naval pathologist. This pathologist was provided certain microphotographs of blood samples that I had taken. The research was not mature to the point that it is now, however, it was mentioned by the pathologist that the condition I was reporting is indeed commonly being observed. This pathologist, to the best of my recollection, attributed the source of the problem to the failure of a particular enzyme within the liver. At this point I cannot recall the name of the specific enzyme. It is nevertheless of great interest to understand that the liver now exists as one of the primary targets of systematic failure within the Morgellons research that is underway.
There are many serious consequences to a liver that is overloaded with toxins. Another example of damage, beyond fatty accumulation, is what is called lipid peroxidation. Lipid peroxidation is caused by the presence of free radicals and it involves the deterioration of fats through an oxidation process. In layman terms, the situation can be equated to that of rancid, or spoiling fats.
The value of knowledge on detoxification of the liver now becomes apparent. The free flow of bile (indicated by peristalsis, or rhythmic contractions of the intestine) may be one of the first conditions to indicate improved digestive activity. Liver detoxification is an important subject in its own right and is likely worthy of serious investigation, study and application by each of us. The purpose here is to indicate simply another aspect of human health that is deeply enmeshed in the path to better health, and that is a smoothly functioning liver.
7. Enzymes. What we are learning here is that the road to better health and the prevention of disease, regardless of the source, requires an integrative process. It may require more effort than many of us are willing to expend. We soon become aware, especially when we seek answers to the serious problems posed above, that we must start to learn how the body actually works. We must start to learn the relationships of one part of the system to another. It is a fascinating and hopefully beneficial process if you are willing to pursue that pathway, but it will not be accomplished without effort on your part. It requires the same of me.
Another simple example of another important relationship is the following. Essentially every chemical reaction that takes place in the body requires the use of enzymes. With an understanding and appreciation of this profound statement, my appreciation for understanding the nature and role of enzymes is now earnest. Enzymes are actually an amazing chemical phenomena; they essentially cause something to happen that would not happen otherwise, and the enzymes themselves are not even changed in the process. They provide an alternative energy pathway to get something done, and with the overall reaction requiring less energy in the process. One analogy given is that of a tunnel through a mountain; you can either climb over the mountain and expend a great deal of energy and effort (and maybe never make it over the top) or you can go through a tunnel if one happens to be there. An enzyme is somewhat analogous to the tunnel though the mountain.
An example of an enzymatic, or catalytic, reaction, is shown in the video segment within this report and above. In this case, hydrogen peroxide is added to iron (ferric) oxide. What the observer sees is a vigorous bubbling reaction. What is occurring in the reaction is that the hydrogen peroxide is being decomposed, or broken down into oxygen and water. The iron in the reaction serves as the catalyst. If you study this reaction long enough, you will find the iron oxide is not changed no matter how long you watch it. It is counter-intuitive, as when we see vigorous bubbles reacting to iron, we expect the iron to visibly change or deteriorate in the process. It does not. But the reaction would not occur without the iron present.
[Edit : Dec 01 2011 :
A drop of one or two degrees in body temperature can have a marked effect on body metabolism and enzyme activity. It is expected that this level of decrease in body temperature could correspondingly decrease enzyme activity on the order of 10% to even 40%97,98. As we have learned of the one to one correspondence between metabolism and enzyme activity, major impairment of our metabolism and functioning is expected with decreased body temperatures. There is an accumulated body of information that indicates that the body temperatures of the general population may now well be lower by this same amount of one to two degrees. This topic exists as a focal point of future research.]
Now that we see more clearly the importance and function of enzymes, we can also understand why a lacking enzyme within the liver might be very serious business. It therefore behooves us to add an additional field of study to our pursuits in biochemistry and health restoration, and this is the study of enzymes. We must learn what enzymes are likely to be involved within the systems that are known to be failing (circulation, respiration, digestion, etc) and what can be done to restore the deficiencies. Once again, I can see no alternative to holistic and integrative medicine and health research in the solution to the problems before us.
In summary, I now see five major challenges before us with the “Morgellons” issue based upon the research that I have conducted to date:
1. The iron within the blood, to a partial degree, is being changed in a way that it no longer binds with oxygen at the normal levels that are expected. This same iron is being used by the organism to sustain its own existence and growth. Diminished oxygen carrying capacity of the blood is therefore expected to occur in coincidence with the severity of the condition.
2. The presence of free radicals are likely to increase in number and extent as a result of the oxidation process mentioned immediately above. Free radicals are known to “wreak havoc in the living system”, as has been mentioned earlier.
3. The altered iron (Fe3+ vs Fe2+) now binds to other molecules, many of them toxic or harmful to health, instead of oxygen as is expected. Several of these alternative ligands are known respiratory inhibitors, and therefore further exacerbate the failures in respiration.
4. The bacteria-like form, which appears to be at the origin of the pathogen, itself binds to oxygen to support its own existence. This is in addition to the consumption of iron already identified. This combination further increases the severity of consequence to human health.
5. The presence of the organism, as encountered, appears to be extensive within the body. It appears to occur within the circulatory, digestive and urinary systems as a minimum.
A few, and only a few, suggestions have been given about how these problems can be approached. These strategies are by no means intended to encompass all needs before us. The will hopefully, however, provide a stepping stone to the further research that exists before us. These problems will never be solved with ignorance or apathy. I encourage you to participate in the process of resolution and accountability, and to support those who act on that same behalf.
Sincerely,
Clifford E Carnicom
Oct 15, 2011
Note: I am not offering any medical advice or diagnosis with the presentation of this information. I am acting solely as an independent researcher providing the results of extended observation and analysis of unusual biological conditions that are evident. Each individual must work with their own health professional to establish any appropriate course of action and any health related comments in this paper are solely for informational purposes and they are from my own perspective.
Clifford E Carnicom
(born Clifford Bruce Stewart, Jan 19 1953)
References:
1. Free Radicals in Biology and Medicine, Dr. P.K. Joseph, PhysicianByte.com
2. Hemoglobin, Wikepedia, July 2011.
3. Hemoglobin, Chemistry Explained, N.M. Senozan.
4. Iron in Cell Culture, Sigma-Aldrich, sigmaaldrich.com.
5. Biochemstry Demystified, Sharon Walker, PhD, 2008, McGraw Hill, p. 264.
6. Iron, University of North Carolina at Pembroke.
7. Determination of Iron with 1,10-Phenanthroline, University of Tennessee, Knoxville, Department of Chemistry.
8. Morgellons : A Discovery and a Proposal, C.E. Carnicom, February 2011.
9. Ibid., Carnicom.
10. IUPAC Gold Book – Fenton Reaction, IUPAC Compendium of Chemical Terminology.
11. Qualitative Analysis Tests, Chemical Identification Tests for Positive Ions, Phil Brown, PhD.
12. Qualitative Analysis Tests, Chemical Identification Tests for Negative Ions, Phil Brown, PhD.13. Easy Chemistry, Josehp A. Mascetta, M.S., 2009, Barron’s Educational Series, pp. 373-375.
13. Definition of Coordinate Covalent Bond, Everything Bio, An All Encompassing Bio Resource.
14. Oxford Dictionary of Chemistry, 2000, Oxford University Press, p 120.
15. A-Z Chemistry, Andrew Hunt, 2003, by McGraw-Hill, p. 101-102.
16. Coordinate Covalent Bond – Definition, www.chemistry-dictionary.com.
17. Modern Biology, Albert Towle, 1999 by Holt, Rinehart & Winston. pp.939, 1108.
18. Biology, Neil A Campbell, PhD. 1993 by Benjamin Cummings Publishing Co. p. 843.
19. Campbell, p 844.
20. Morgellons : A Discovery and a Proposal, Carnicom.
21.Free Radicals in Biology and Medicine, Dr. P.K. Joseph.
22. Ibid., Joseph.
23. Morgellons, A Fourth Match, Carnicom, 2008.
24. Morgellons: The Wine-Peroxide Test, Carnicom, 2008.
25. Morgellons: The Extent of the Problem, Carnicom 2010.
26. Morgellons: A Status Report, Carnicom, 2009.
27. Chemistry Made Simple, John T. Moore, Ed. D., 2004, Broadway Books, p 134-135.
28. Foundations of College Chemistry, Morris Hein, 1996 by Brooks/Cole Publishing Co., p 157-158.
29. American Druggist, Volume 22, Google Books, p 197.
30. Oxidation of Ascorbic Acid, Chemistry Comes Alive, Volume 5.
31. Growing Metal Crystals; Electrolysis of Metal Salts, Derek’s Mundane Web.
32. Electroplating, Electrochemistry Encyclopedia, Case Western Reserve University, Ernest B. Yeager Center for Electrochemical Sciences.
33. General Chemistry, Linus Pauling, Dover Publishing, 1988, pp. 512-520.
34.Can Iron Ore Be Extracted by Electrolyis?, Yahoo Answers, Malaysia.
35. Iron (III) Oxide, Journal of Chemical Education, Vol 78, No. 10, October 2001.
36. Catalytic Decomposition of Hydrogen Peroxide and 2-chlorophenol with iron oxides, Water Research, Vol. 35, Issue 9, June 2001, pp.2291-2299. Abstract.
37. Ligand, Wikipedia.
38. Thinkwell Chemistry, Transition Elements, Dr. Dean Harmon and Dr. Gordon Yee.
39. Spectrochemical Series, ChemWiki, University of California at Davis.
40. Ibid., Ligand, Wikipedia.
41. Transition Metals and Coordination Chemistry, Chapter 20 Notes, University of Washington, p.5.
42. Ibid, Walker., Chemicals That Impede Hemoglobin Functions – Poisons. P 272
43. Ibid., Thinkwell Chemistry.
44. Altered Blood, Carnicom, Jun 2011.
45. Definition of Methemoglobinemia, MedicineNet.com.
46. Causes and Clinical Significance of Increased Methemoglobin, Asociacion Espanola de Farmaceuticos Analistas., www.aefa.es
47. Ibid., AEFA.
48. Ibid., AEFA.
49. Ibid., Walker, p. 27.
49. Respiration, Royal Society of Chemistry, www.rsc.org
50. Energy Changes in Chemical Reactions, Avogadro Web Site, www.avogadro.co.uk
51. Ionization Potentials of Atoms and Atomic Ions, Handbook of Chemistry and Physics, 82nd Edition, CRC Press, p 10-175.
52. Properties of Atoms, Radicals and Ions, Table 4.11 Bond Dissociation Energies, Department of Inorganic Chemistry, University of Buenos Aires.
53. Chemistry, The Central Science, Theodore L. Brown, PhD, 2006 by Pearson Education – Prentice Hall, p. 1036.
54. Morgellons: A New Classification. Carnicom. Feb. 2010.
55. How Some Bacteria May Steal from their Human Hosts, Science Daily, Aug 2008.
56. Do Bacteria Affect the Rusting of Iron?, Douglas Bintzler, www.ehow.com
57. Iron Bacteria,BioVir Laboratories, Inc., www.biovir.com.
58. Bacteriology – Chapter Three – Nutrition, Growth and Energy Metabolism, Dr. Alvin Fox, Microbiology and Immunology On-line, University of South Carolina School of Medicine.
59. Siderophore Uptake in Bacteria and the Battle for Iron with the Host; A Bird’s Eye View, Chu BC, Biometals, Aug 23. 2010., Abstract.
60. Iron Metabolism in Pathogenic Bacteria, Colin Ratledge, Annual Review of Microbiology, Vol 54. p. 881-941, Abstract.
61. Iron Metabolism Bacteria, Ken Burnside, www.ehow.com
62. Microorganisms Pumping Iron; Anaerobic Microbial Iron Oxidation and Reduction, Karrie A Weber, Nature Reviews Microbiology, Oct. 2006, Abstract.
63. Free Iron in Bacteria, Jim Imlay PhD, Department of Microbiology, University of Illinois, Urbana-Champaing, Society for Radical Biology and Medicine.
64. Topic 6, Coordination Compounds, Georgia Tech University, Chemistry and Biochemistry, www.chemistry.gatech.edu.
65.Enterobactin, Wikipedia.
66. Siderophore Electrochemistry: Relation to Intracellular Iron Release Mechanism, Proceedings National Academy of Science, Vol. 75, No 8, pp. 3551-3554. Aug 1978, Chemistry.
67. Carnicom Institute, www.carnicominstitute.org
68. A Spectrofluorimetric Sensor Based on Grape Skin Tissue for Determination of Iron(III), Minghui Zhang, Bulletin Chemical Society of Ethiopia 2010 24(1), 31-37.
69. The Role of Iron and Tion in Discoloration of Berry and Red Beet Juices, Heikki Pyysalo, Zeitschrift Fur Lebensmitteluntersuchung Und – Forschung A, Volume 153, Number 4, 224-233. Abstract.
70. Blue Metal Complex Pigments Involved in Blue Flower Color, Kosaku Takeda, Proceedings of the Japan Academy, Series B, Physical and Biological Sciences, Vol 82 (2006), No. 4, p 142-154. Abstract.
71. Determination of Anthocyanins in Red Wine Using a Newly Developed Method Based on Fourier Transform Infrared Spectroscopy, A. Soriano, Food Chemistry, Vol. 104, Issue 3, 2007, P1295-1303. Abstract.
72. Iron-Polyphenol Complex Formation and Skin Discoloration in Peaches and Nectarines, Guiwen Cheng, Journal of the American Society for Horticultural Science, Vol 122, Jan. 1997, p. 95-99.
73. Robbins Pathologic Basis of Disease, Ramzi S. Cotran, M.D., 1989, W. B. Saunders Company, 4th Edition
74. Ibid., Cotran, p 3.
75. A Mechanism of Blood Damage, C.E. Carnicom, Dec. 2009.
76. Enzymes, Cliffs Notes, www.cliffsnotes.com
77. Ibid., Walker. p 185.
78. Micronutrient Information Center, Linus Pauling Institute., Oregon State University.
79. Ibid., Johnson.
80. Antioxidants, Better Health Channel, Victorian (Australia) State Government.
81. Ibid., Walker, p 169.
82. Ibid., Linus Pauling Institute.
83. A Simple Quantitative Bedside Test to Determine Methemoglobin, Fathima Shihana, BSc, South Asian Clinical Toxicology Research Collaboration, Annals of Emergency Medicine, Vol. 55, No 2. Feb 2010.
84. Acidity, Disease and Cancer, Health News, http://www.healthnews-nz.com
85. Ibid., Cotran, p. 4.
86. Body Acidity, Disease Prevention and More About Aspartame, Stephen Sampson, www.associatedcontent.com.
87. Morgellons: A Discovery and A Proposal, C.E. Carnicom, Jun. 2011.
88. Morgellons: In the Laboratory, C.E. Carnicom, Jun. 2011.
89. Biochemistry, Philip Kuchel, PhD, (2009, McGraw-Hill, 32).
90. Biochemistry for Dummies, John Moore, EdD, (2008, Wiley Publishing, 29).
91. Brown, Steven; Chemistry 102a Laboratory Manual, Kendall Hunt Publishing Company, 1996., p 125.
92 Ibid., A Discovery and A Proposal, C.E. Carnicom.
93. Ibid., Cotran, p. 12.
94. Ibid., A Discovery and A Proposal, C.E. Carnicom.
95. Ibid., Linus Pauling Institute.
96. Ibid., Morgellons: In the Laboratory, C.E. Carnicom.
97. Temperature Effects – Introduction to Enzymes, Worthington Biochemical Corporation, www.worthington-biochem.com
98.The Cold Body Page, http://www.mall-net.com/mcs/coldbody.html.