Tertiary Rainwater Analysis : Questions of Toxicity
Clifford E Carnicom
Nov 08 2015
ABSTRACT
This paper presents evidence of a chemical signature that exists within an analyzed rain sample that is characteristic of known toxins and pesticides. The method of analysis used is that of mid-infrared spectroscopy. Specifically, certain functional groups involving sulfur, nitrogen, phosphorus, oxygen, and halogens have been identified in the analysis. It is recommended that the investigation be duplicated by independent researchers to determine if an environmental hazard does exist. If these results are verified to be positive, the source of the contaminants is to be identified and eliminated from the environment.
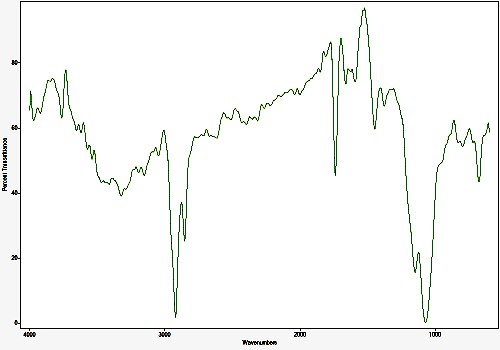 Infrared Spectrum of Concentrated Rain Water Sample
Infrared Spectrum of Concentrated Rain Water Sample
(Aqueous Influence Removed)
The original rainwater sample volume for this analysis is approximately 3.25 liters. The sample was evaporated under mild heat to approximately 0.5% of the original volume, or about 15 milliliters. The sample has previously been shown to contain both aluminum, biological components, and a residue that appears to be an insoluble metallic or organometallic complex. The target of this particular study is that of soluble organics.
The organic infrared signal within the solution is weak and difficult to detect with the means available; it is further complicated by being present in aqueous solution. The aqueous influence was minimized by making an evaporated film layer on a KCl cell; the transmission mode was used. The signal is identifiable and repeatable under numerous passes in comparison to the reference background.
The primary conclusion from the infrared analysis is that a core group of elements exists within the solution; these appear to include carbon, hydrogen, nitrogen, sulfur, phosphorus, oxygen and a halogen. The organic footprint appears to be weak but detectable and dominated by the above heteratoms.
As further evidence for the basis of this report, qualitative tests for an amine (nitrogen and hydrogen), sulfates and phosphates (sulfur, oxygen and phosphorus) have each produced a positive test result. A qualitative test for a halogen in the concentrated rainwater sample has also produced a positive result; the most likely candidate at this point is the chloride ion. All elements present have therefore been proven to exist at detectable levels by two independent methods.
This grouping of elements is distinctive; they essentially comprise the core elements of many important, powerful and highly toxic pesticides. For example, three sources directly state the importance of the group above as the very base of most pesticides:
“In pesticides, the most common elements are carbon, hydrogen, oxygen, nitrogen, phosphorus, sulfur and chlorine”.
Pesticide Residues in Food and Drinking Water : Human Exposure and Risks, Dennis Hamilton, 2004.
“We can further reduce the list by considering those used most frequently in pesticides: carbon, hydrogen, oxygen, nitrogen, phosphorus, chlorine, and sulfur”.
Fundamentals of Pesticides, A Self-Instruction Guide, George Ware , 1982.
“Heteratoms like fluorine, chlorine, bromine, nitrogen, sulfur and phosphorus, which are important elements in pesticide residue analysis, are of major interest”.
Analysis of Pesticides in Ground and Surface Water II : Latest Developments, Edited by H.J. Stan, 1995.
It is also true that phosphate diesters are at the core of DNA structure and that many genetic engineering procedures involve the splitting of the phosphate diester complex.
The information provided above is sufficient to justify and invoke further investigation into the matter. The sample size, although it was derived from an extensive storm over several days in the northwest U.S., is nevertheless limited and quite finite after reduction of the sample volume. The residual insoluble components (apparently metallic in nature) are also limited in amount and more materials will be required for further analysis. The signal is weak and difficult to isolate from the background reference; concentration level estimates for elements or compounds (other than that of aluminum which has been assessed earlier) is another entire endeavor. Systematic, wide-area, and long term testing will be required to validate or refute the results. All caveats above aside, it would seem that the duty to address even the prospect of the existence of such toxins in the general rainfall befalls each of us. It would seem wise that this process begins without delay.
There are a few additional comments on this finding that need be mentioned.
The first of these is the issue of local and regional vs. a national and international scope of consideration. It is understood that pesticides or compounds similar in nature are a fact of our environment, and that considerable awareness and effort is in place to mitigate their damage over decades of use. Organic farming and genetically engineered crops are two very divergent approaches to reconciliation with the impact of environmental harm, and they are shaping our society and food supply in the most important ways manageable. Given that the pesticide industry exists, regardless of our varying opinions of merit or harm, I think that it is fair to say that we generally presume that pesticides are under some form of local control. Our general understanding is that pesticides are applied at ground or close to ground level and are intended to be applied to a specific location or, at most, a region within a defined time interval.
The prospect, even I daresay, the hint, of pesticide or pesticide-like compounds in rainfall is more than daunting. It seems immediately necessary to consider what scale of operation would support such toxins finding their way into the expanses of atmosphere and rainfall? For the sake of the general welfare, I think we should all actively wish and seek to disprove the findings within this report. I will not hesitate to amend this report if honest, fair and accurate testing bears out negative reports over an adequate time period, and my motive never includes sensationalizing an issue. This is one test, one time, one place, with limited means and support in the process. I cannot disprove the results at this time and I have an obligation to report on that which seems to be case, uncomfortable as it might be. It is not the first time that I have been in this situation, and judging from the changes in the the health of the planet that have taken place, it is unlikely to be the last. The sooner that the state of truth is reached, the better we shall all be for it in any sense that is real.
The second comment relates to the decline of the bee population. Bees are an indicator species, the canary in the mine, as it were. The bees and the amphibians have both been ringing their alarm for some time now, and we best not remain passive about finding the reasons for decline. A minimum of 1/3 of our agricultural economy, and that means food, is dependent upon the bee population for its very existence. This is no trifling matter, and we all need to get up to speed quickly on the importance of this issue, myself included.
Suffice it to say that compounds of this nature, i.e, historical pesticides like organophosphates and the purported safer and more recent alternatives (e.g., the neonicotinoids), have a very close relationship to the ongoing and often ambiguous studies regarding bee Colony Collapse Disorder (CCD). From my perspective, it would seem prudent to eliminate the findings of this report as a contributing cause to the problem as promptly as possible. If that can not be done so readily, then we may have a bigger problem on our hands than is imagined.
One of the interesting side notes is that the elements and groups identified as candidates for investigation actually seem to overlap between the neonicotinioids and the organophosphates. This includes the nitrogen groups that characterize the neonicotinoids and the phosphate esters that characterize the organophosphates. If such a combination were at hand, this would seem especially troublesome as both forms remain mired in controversy, let alone any combination thereof.
The third and final comment relates to the toxicity of these compound types in general. It is not just an issue about bees or salamanders. These particular compounds have a history and effects that are not difficult for us to research, and we should become aware of their impacts upon the planet quickly enough. Many of us already are. The fact is that organophosphates have their origins as nerve gas agents in the pre-World War II era, and in theory their use has been reduced but hardly eliminated. Residential use is apparently no longer permissible in the United States, but commercial usage still is. This raises questions on what real effect any such “restrictive” legislation has had.
The neonicotinoids are promoted as a generally safer alternative to the organophosphates, but they are hardly without controversy as well. They too have strong associations with CCD in the research that is ongoing. They also are neuro-active insecticides.
It would seem to me that we all have a job to do in getting up to speed on the source, distribution and levels of exposures to insecticide and insecticide related compounds. A greater awareness of toxins in our environment, in general, also seems in order. If our general environment has been affected to a degree that has avoided confrontation thus far, then we need to face the music as quickly as possible. I trust that we understand the benefits of both rationality and aggressiveness when serious issues face us, and this may be another such time. I hope that I will be able to dismiss this report in due time; at this time, I cannot.
Sincerely,
Clifford E Carnicom
Nov 05, 2015
Born Clifford Bruce Stewart
Jan 19, 1953
Additional Notes:
The preliminary functional group assignments being made to the absorption peaks at this time are as follows (cm-1):
~ 3322 : Amine, Alkynes (R2NH considered)
~ 2921 : CH2 (methylene)
~ 2854 : CH2 (methylene)
~1739 : Ester (RCOOR, 6 ring considered)
~1447 : Sulfate (S=O considered)
~1149 : Phosphate (Phosphate ester, organophosphate considered)
~1072 : Phosphine, amine, ester, thiocarbonyl
~677 : Alkenes, aklynes, amine, alkyl halide
The assignments will be revised or refined as circumstances and sample collections permit, however, as a group they appear to provide a distinctive organic signature. A structural model may be developed at a future date.
Some chemical compounds which may share some similar properties to that under consideration here include, for example, (not all elements included in any listed compound; only for reference comparison purposes):
p-chlorophenyl (3-phenoxypropyl)carbamate
N-(1-naphthylsulfonyl)-L-phenylalanyl chloride
2,2,2-trichloroethyl 2-(2-benzothiazolyl)dithio-alpha-isopropenyl-4-oxo-3-phenylacetamido-1-azetidineacetate
cytidine monophosphate
diiodobis(triphenylphosphine)nickel(II)
per :
SDBSWeb : http://sdbs.db.aist.go.jp (National Institute of Advanced Industrial Science and Technology, Nov 06 2015)



