CDB Lipids : An Introductory Analysis
Clifford E Carnicom
Mar 12 2015
Edited May 29 2016
Note: I am not offering any medical advice or diagnosis with the presentation of this information. I am acting solely as an independent researcher providing the results of extended observation and analysis of unusual biological conditions that are evident. Each individual must work with their own health professional to establish any appropriate course of action and any health related comments in this paper are solely for informational purposes and they are from my own perspective.
An introductory qualitative and analytical analysis of certain lipids that have been extracted from the cross-domain bacteria (CDB), as they are designated on an interim level by this researcher, has been made. Lipids are a primary biological molecule within any living organism and future studies of this component will be of the greatest importance.
Several major characteristics have been identified using modest means and methods, and the results bring to the forefront additional unusual properties of the organism under study with respect to the so-called “Morgellons” condition. There are, potentially, several important health implications that arise from this recent work; these health factors are in complete accord with the historical record of discovery and examination that is available on this site. This paper will be relatively brief in coverage but it will, hopefully, serve to reiterate certain themes and directions of research that remain to be confronted by society and that are deserving of appropriate support and resources.
The primary characteristics or factors that have been identified in the course of this study are:
1. The lipids from the CDB appear to be highly non-polar in nature.
2. The lipids have a relatively high index of refraction.
3. The lipids appear to be composed, in the main, from long chain poly-unsaturated fatty acids.
4. The lipids appear to support combustion (i.e., oxidation) with ease.
5. The lipids appear to react readily with the halogens, such as iodine.
6. The visible light spectrum of the lipid – iodine reaction is unique and it serves as an additional means of identification. Peak absorbance of the reaction is at approximately 498 nanometers.
7. A significant portion of the extracted lipids is expected to originate from the membranes of the CDB.
8. Endoxtoxins within the CDB are suspected to exist and this subject remains as a serious prospect for research in the future.
These characteristics will now be discussed in greater detail to formulate a general but composite assessment of the lipid character, as well as a reference to certain health impacts that are necessary to consider.

Variable Solubility of the Lipids as it Relates to Polarity
Polarity is a defining property of a molecular structure, and it is a measure of the distribution of charges within a molecule. Non-polar molecules are generally symmetric in their nature with a tendency toward an equal and symmetric distribution of charges. Polar molecules, in contrast, are usually of an asymmetric nature with the charges on the molecule unevenly distributed. Information on polarity, therefore, provides some generalized nature as to the form or nature of the molecule or substance under study.
In this photo, The lipids are mixed with a mildly polar solvent in the tube to the left in the photo; a clear separation remains after settling. In contrast, the lipids dissolve much more readily in a highly polar solution to the right in the photograph.
The significance of this result is as follows:
Fatty acids are a dominant component of many lipids. They are comprised of a carboxyl group that is attached to a hydrocarbon chain. The length of this chain can vary depending upon the particular fatty acid that is involved. The carboxyl group is polar in nature and therefore the charge distribution on that particular functional group is asymmetric. The carboxyl group is also acidic in nature and this is the origin of the name of fatty acids that is attached to this common lipid structure.
The hydrocarbon chain that is attached to the carboxyl group is generally of a non-polar nature, and it serves to counteract the polar effect from the carboxyl group. Therefore, the more non-polar the lipid is, the more likely it is that the hydrocarbon is of relative greater length. A very long hydrocarbon chain (non-polar) will tend to dominate the character of the molecule in this case and ultimately make the molecule less polar.
This relationship between the polarity of and the length of the attached hydrocarbon chain provides our first useful interpretation as to the structure of the lipid molecule. Some lipids are more or less polar than others; a highly polar lipid is indicative of lengthy hydrocarbon chains within the fatty acid. The longer the fatty acid is, the more complex the lipid structure or interactions with other molecules is likely to be. The structure of any molecule is of the highest importance, as one of the dogmas of biology is that structure determines function. We are after both, structure and function, and usually in that same order.
A couple of examples of short vs. long chain fatty acids follows; it can be seen that the differences in form and structure can be substantial:
Short Chain Fatty Acids
Image Source : intechopen.com
The specific conclusion in this case is that we are more likely to be dealing with a lipid form that contains more extensive hydrocarbon chains.
The next topic of interest concerns the index of refraction. The index of refraction is a measure of the ability of a substance to bend a light wave that passes through it. It is also a measure of the speed of light though that same material. It is also an important defining physical property of a substance, and its measurement can be made with relative ease and modest cost. Tables of the index of refraction for a wide variety of substances, including lipids and oils are readily available for comparison purposes.
The index of refraction for the lipids under examination measures at 1.487 as the average between two different samples. The instrument has been calibrated with numerous comparison oil samples and is performing accurately and reliably. The estimated error of the measurement is +/- .001.
The measurement of 1.487 is a relatively high index of refraction, especially as far as oils are concerned. This higher measurement also leads to interpretations of significance as we shall soon discover.
There is a relationship between the index of refraction and the degree of saturation within a fatty acid or lipid. The saturation level (i.e., saturated vs unsaturated) property of a lipid is also a very important characteristic as it expresses itself in terms of the the bond types within the molecule; this is an additional aspect of structure that we have declared as our pursuit.
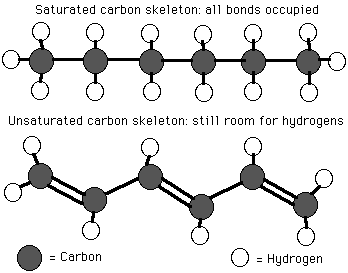
Let us begin with the definitions for saturated vs. unsaturated fats. A saturated fat is one in which a full complement of attached hydrogen atoms exists. A saturated fat contains only single bonds between the carbon atoms. An unsaturated fat, in contrast, has double (or higher) bonds between the carbon atoms, and there will be fewer hydrogen atoms attached as a result. Let us present a couple of images to clarify the difference between saturated and unsaturated fats.
An example of a saturated vs. an unsaturated fat
image source : staff.jccc.net
In addition, a distinction should be made between mono-unsaturated fats and poly-unsaturated fats. In essence, a mono-saturated fat has a single double carbon bond within the hydrocarbon chain and a poly-unsaturated fat has more than one double carbon bond within the chain. The image below shows this difference
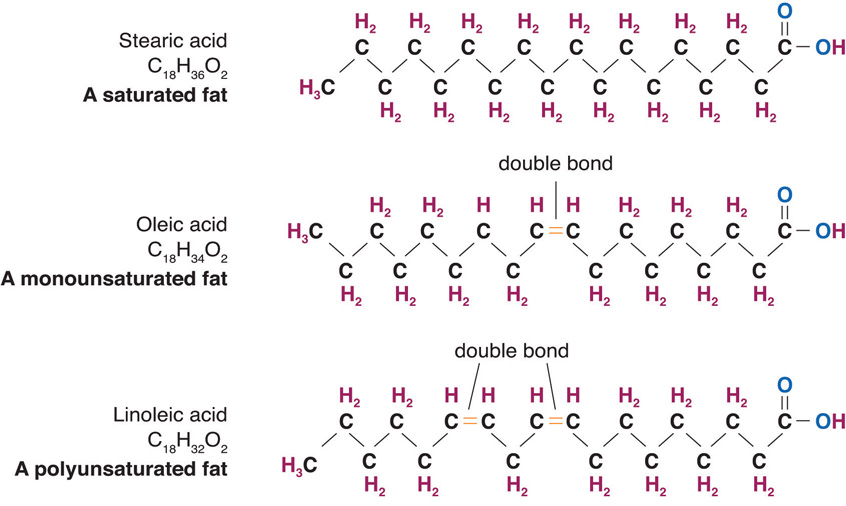
The top image shows another example of a saturated fat.
The lower two images show the distinction between monounsaturated and polysaturated fats.
Notice the number of number of double carbon bonds present in the latter examples.
image source : 2012books.lardbucket.org
As information is gained, let us never lose sight of the end goal:The more that can be understood about the structure of a biological molecule, the closer that we are towards learning about the behavior, interaction and function of that molecular structure. This information is a prerequisite toward the design of effective mitigation strategies. While much of this pursuit remains in our future, we nevertheless can report the modest levels of progress as they occur, albeit under restricted conditions.
Now that we understand the variations of saturation within fats and oils (lipids), let us return to something that can be measured to give us information about the state of saturation within a lipid. Once such measurement is the index of refraction, as has been referred to above.
It will be found in the literature that that there is a ‘relationship’ between the degree of saturation in a fat and the ‘iodine number’. The iodine number is a measure of the level of absorption of iodine by fats, and this number can be used in turn to infer the degree of saturation by that same lipid or fat. The method is commonly used in the food industry to determine the quality of fats. The degree of fat saturation is a variable of high interest within the food industry as it affects the spoilage rate and this in turn affects the economics of the food industry. There are many important reasons to understand the qualitative characteristics of lipids beyond our immediate interest in the ‘Morgellons’ issue.
Determination of the iodine number is a more demanding laboratory method and it requires additional time, protocols and reagents in comparison to alternative methods that have developed within this study.
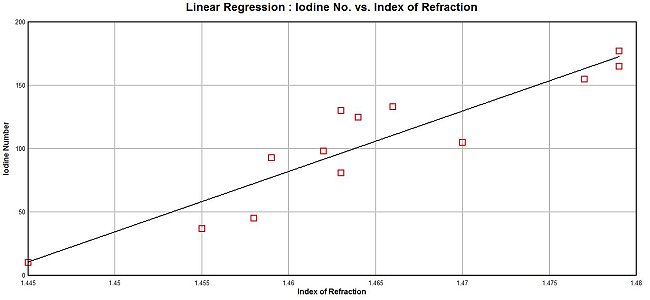
There is, however, a more accessible method to fulfill our immediate need, and that is to get some sense of the likely saturation level of this particular lipid. It will be found, with study, that there is also a relationship that can be established between the index of refraction of an oil and the iodine number of that same oil. An increase in the iodine number is indicative of a higher unsaturation level and in parallel it will be found that a higher index of refraction is strongly correlated with a higher iodine number. We are able, therefore, to make an equally viable interpretation of the saturation (i.e, unsaturation as well) level with the use of the index of refraction as our primary dependent variable. Ultimately, a higher iodine number estimate will indicate a higher level of unsaturation within the lipid. Such a relationship has been researched and established as presented below.
Several different oil types have been investigated and the correlation between the index of refraction is reasonably strong (r = 0.92, n = 13). The accuracy of the refractometer in use has been included as a part of the study. The result of this work is that a viable method to estimate the level of relative saturation from a direct measurement of the index of refraction of the lipid under study now exists.
The application of the linear regression model to the measured index of refraction (1.487) yields an estimate for the iodine value as 218. This magnitude for the estimated iodine value is extremely high and it is significant in its own right.
The conclusion to be reached from this iodine value is meaningful. This stage of the study indicates that the character of the lipid is more likely to be that of a highly poly-unsaturated lipid. This result is corroborative with the first interpretation of a relatively lengthy fatty acid chain within the lipid structure. These two interpretations are mutually supportive of one another. This means that the lipid hydrocarbon chains are more likely to be lengthy with several double carbon bonds along the chain. This, in turn, will affect the structure as double bonds cause a bend to take place in the hydrocarbon chain. Several double bonds would only enhance that feature further.
In addition, double bonds within a hydrocarbon chain have another likely and important result. They are much more likely to produce chemical reactions. Two likely candidates for reaction are oxygen and the halogens. Lipids with a high iodine value are more subject to oxidation and therefore have a greater likelihood of becoming rancid (spoiled). High iodine level lipids are also more likely to produce free radicals. Lastly, highly polyunsaturated lipids are more likely to polymerize (i.e, ‘plasticize). Each of these impacts offer the prospect of additional harm to the body, and great attention to the effects of oxidation and free radicals has been given in the history of research on this site.
There is a wealth of information that is available on the health risks associated with polyunsaturated fats. The following citations are a couple of representative examples of the issues involved, the first from a lay standpoint and the second from the Commission of European Communities:
 Source : Reports of the Scientific Committee for Foods, Commission of European Communities
Source : Reports of the Scientific Committee for Foods, Commission of European Communities
Readers may recall the extensive attention that has given within this site to the role that antioxidants can play in the mitigation of excessive oxidation to the body. Those discussions, once again, appear to be especially relevant in the amelioration of the harmful influences of polyunsaturated fats. The impact of halogens to the thyroid and metabolism have also been extensively discussed on this site and we will return to that topic later in this paper as well.
The issue of oxidation in combination with combustion tests should now be raised. The tests, at this stage of investigation, indicate that this particular species of lipids may be highly subject to the process of oxidation. The purity of the sample can not be quantified at this point since there may be other compounds present within the lipid samples. However, all indications are that the character of the lipids is somewhat unusual with respect to oxidation and, for that matter, combustion.
The lipids that have been extracted ignite easily, as is shown in the photograph below on the left side:
In this case, the method involves placing a small amount of the lipids into a watchglass with a small piece of paper acting as a wick. The lipids burn easily and steadily under these conditions, and the behavior is somewhat akin to lamp oil. Due to the biological and apparent polyunsaturated nature of the lipids, a comparison might be made with whale oil, which was an important source of fuel in earlier times. There is no suggestion here that the lipids are chemically identical to whale oil by any means, however, the fish oils and whale oil share many interesting properties of the highly polyunsaturated fats. The photograph on the right shows the wick remaining at the end of combustion; this demonstrates that the oil itself is the primary source of fuel within combustion. The last photograph shows an inclusive example of the failure of any of the other tested lipids or oils to support direct combustion.
Combustion goes hand in hand with oxidation; something that burns oxidizes. It is of interest that of all the other oils tested under similar conditions (approximately 8 varieties of varying degrees of unsaturation), only the lipids under examination here showed any ease of combustion at the level shown within the photographs. Along with the highest index of refraction found within the group that has been examined, the dramatic display of combustion of the sample further reinforces the case for a lipid that is highly unsaturated and thus prone to excessive oxidation. This finding is once again corroborative of the extensive case for excessive oxidation within the body that occurs in association with the ‘Morgellons’ condition; readers may also recall the lengthy discussions on the apparent marked oxidation of iron within during the examination of blood samples. All signs of the accumulated research indicate that excessive oxidation within the body is one of the most likely outcomes expected to be found within any future studies of the ‘Morgellons’ condition. Preliminary data from early questionnaires submitted to the public also strongly indicates this same result.
There are at least two primary forms of lipids in the body, one for storage of energy within the cells and another within the membranes of the cell, where they act to to encapsulate and protect the cell. Saturated fats are more likely to be associated with the storage of energy internal to the cell and unsaturated fats are more likely to be associated with the membranes of a cell . Phospholipids are a very important class of lipids that are found within the cell membranes. The degree of unsaturation within phospholipids varies, with one or both tails having double carbon bonds (the site of oxidation). An image of a representative phospholipid follows:
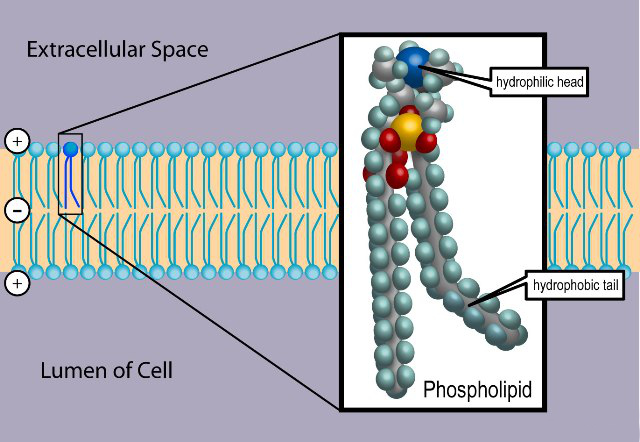
Phospholipid within a Cell Membrane
Source : wikipedia.com
The oxidation of lipids is referred to as lipid peroxidation, and it is especially prone to occur with polyunsaturated lipids, as we appear to have in this case. Phospholipids (a bi-layer) are a major constituent of cell membranes, and the oxidation of these lipids subsequently causes damage to the cell. Lipid peroxidation is essentially the theft of electrons from the lipids in the membranes and it occurs as a free radical chain reaction. The oxidation occurs when there is an excess availability of free radicals, or reactive oxygen species. The point of oxidation will be the location of the double bond, which occurs at the bent location within the unsaturated fatty acid tail, as shown in the picture above. An illustration of the lipid peroxidation reaction is shown below; notice the site of activity at the carbon double bond:
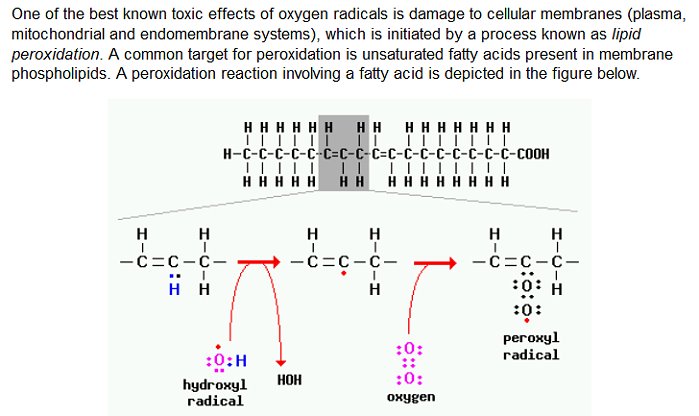
Source : Colorado State University
It appears to be the case at this point that the CDB contain within them a highly polyunsaturated fat and/or fatty acids, most likely to occur within the membranes of the CDB, and that the CDB may therefore be subject to, or result in, lipid peroxidation in the presence of free radicals. This process, once started, is a chain reaction and is only terminated in the presence of appropriate antioxidants, such as Vitamin E, glutathione peroxidase, transferrin (binding free iron), enzymes (such as catalase), in addition to others[see Robbins above]. As shown within earlier culture trials, Vitamin C and NAC (N-acetyl cysteine acting as a glutathione precursor) may show themselves to be effective antioxidants as well. The issue of oxidants vs. antioxidants has emerged earlier within the research and this information remains available to review. Those seeking therapeutic protocols dependent upon oxidizing protocols vs. antioxidant protocols may wish to examine further the fundamental differences that are apparent within the scientific literature. Each individual must , of course, seek health consultation that is appropriate to their individual needs.
Another more complete description of lipid peroxidation comes from Robbins Pathologic Basic of Disease, 4th Edition, where the following sequence is described:
“Lipid peroxidation is one well-studied…mechanism of free radical injury. It it initiated by hydroxyl radicals, which react with unsaturated fatty acids of membrane phospholipids to generate organic acid free radicals, which in turn react quickly with oxygen to form peroxides. Peroxides themselves then act as free radicals, initiating an autocatalytic chain reaction, resulting in further loss of unsaturated fatty acids and in extensive membrane damage”
To reiterate the attention that has been given in the research to the oxidation and antioxidant issues in the case of ‘Morgellons’, please recall some of the earlier papers (this paper included) that complement this discussion:
Morgellons : A Discovery and a Proposal – February 2010
Morgellons : Growth Inhibition Confirmed – March 2010
Morgellons : The Extent of the Problem – June 2010
Morgellons : In the Laboratory – May 2011
Morgellons : A Thesis – October 2011
Morgellons : The Breaking of Bonds and Reduction of Iron – November 2012
Amino Acids Verified – November 2012
Morgellons : A Working Hypothesis : Part I – December 2013
Morgellons : A Working Hypothesis : Part II – December 2013
Morgellons : A Working Hypothesis : Part III – December 2013
Growth Inhibition Achieved – January 2014
Biofilm, CDB and Vitamin C – April 2014
CDB : General Characteristics (In Progress) – July 2014
CDB Lipids : An Introductory Analysis – March 2015
Lipid peroxidation is a complex area for study, however, the importance of doing so can be understood from the following statement by Marisso Repetto, from the Institute of Biochemistry and Molecular Medicine, Argentina:
“Currently, lipid peroxidation is considered [as one of] the main molecular mechanisms involved in the oxidative damage to cell structures and in the toxicity process that lead[s] to cell death.”
The complete paper is detailed but insightful, and it demonstrates the extensive research that is now available on the subject of lipid peroxidation. The paper in its entirety may be accessed here.
Let us introduce an observed reaction with one of the halogens, in this case, iodine. The reaction is shown below on the right hand side, and in comparison to a negative reaction with vegetable oil on the left. Similar to the case of combustion from above, the CBD lipids under study are the only lipids (of approximately eight in comparison) that have displayed this pronounced reaction with iodine. It appears to be a unique, important and characteristic reaction.
It is understood that iodine reacts with lipids; in fact, this is the very basis of the ‘iodine number’ method and it is used as a measure of the unsaturation level of the lipid. The higher the iodine level, the higher the level of unsaturation in the lipid. We have already discussed the relationship between the iodine number and correlation with the index of refraction, and we have very good reason to suspect a very high level of unsaturation within the lipids examined.
What is under discussion here is the formation of a bright red colored iodine complex which, thus far, presents itself only within this particular lipid form, at least in relation to numerous sample types that it has been compared with. The colored complex reaction formed is, in itself, worthy of continued chemical analysis and investigation. This reaction has not occurred in like fashion to any other lipid samples examined thus far. The nature of the complex is not completely understood at this time; the consideration of an iron-lipid-iodine or transition metal complex, however, is extremely high on the list of possibilities.
What can be concluded from visible light spectroscopy, however, is that the colored complex formed once again assures us that we are dealing with a structure that contains numerous double carbon bonds. Visible light spectroscopy is highly dependent upon what is termed conjugation; conjugation is a molecular structure that is based upon alternating single and double carbon bonds. The greater the degree of conjugation, the longer the wavelength of the color that will be absorbed. An example of a highly conjugated form is as follows:
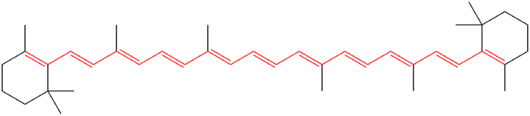
An example of a conjugated structure within a chromophore
(portion of a molecule that absorbs color).
Source : wikipedia
Notice the numerous alternating single and double bonds in the above structure. Chromophores are especially likely to form with compounds that involve the transition metals, such as iron. The color of the complex lends itself well to visual light spectrometry and a spectral plot of the CDB complex formation in the visible light range is shown below:
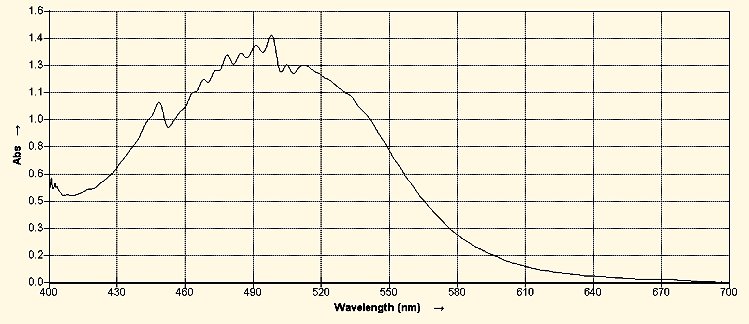
Visible Light Spectrum of the CDB Lipid-Iodine Complex
The peak absorbance occurs at approximately 498 nanometers. This spectral examination of the lipid-iodine complex is an important identification method to establish the presence or existence of this particular CDB lipid form.
The identification of an iron-lipid-iodine complex is further substantiated with tests for the detection of iron using 1,10 phenanthroline reagent in combination with the lipids in a mildly polar solution. These initial tests are weak in color but nevertheless positive for the presence of the Fe+2 ion within the CDB lipids. This finding is in coincidence with the paramount conclusion of significant Fe+2 iron use and metabolism by the CDB, as it has been discussed extensively within earlier papers.
The impact of halogens upon the body has been discussed extensively in earlier work and it will not be repeated here. Readers are referred to the paper entitled Morgellons : A Working Hypothesis (esp. Parts II & III) for the important effects and toxicity potential discussed therein.
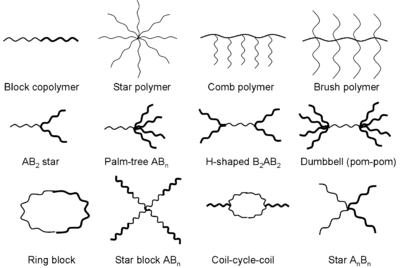
The next topic of importance to discuss is that of polymerization. A polymer is a molecular structure that is composed of many repeating smaller units. They can be either synthetic or natural, and they usually have a large molecular mass compared to that of the basic structural unit. Latex and Styrofoam are examples of both a natural and a synthetic polymer. The architecture and length of the polymer chains strongly affect the physical properties of the polymer, such as elasticity, melting point, and solubility, amongst others. A diagram of various structural forms is shown below:
The reason that polymerization is relevant here is that unsaturated lipids are prone to polymerization. The higher the degree of unsaturation, the more likely that polymerization will take place. This is due to the oxidation at the double carbon bonds that have been brought to attention repeatedly here. A familiar example of polymerization to many of us is with the use of linseed oil. Linseed oil is a highly unsaturated lipid that is applied to furniture as a protective coating; this is one of the so-called “drying oils”. As this type of oil weathers (or oxidizes), it will form a harder and protective coating over the wood surface. This is an excellent example of the oxidation of a highly unsaturated oil, or lipid, that produces a polymer. As mentioned, polymers can vary widely in their physical properties, and the plastics are an excellent additional example of synthetic polymers. Oil paints that artists use are another example of the “drying oils” that share these same characteristics.
It appears that the probability of polymerization for the CDB lipid complex appears to be high at this point, as all of the prerequisite characteristics appear to be in place. It appears to be highly unsaturated and therefore subject to oxidation as has been detailed above. This places us on the alert that the CDB lipids may be a candidate to produce polymers which, in general, would be anticipated to cause harm if internal to the body.
With respect to lipid discovery and extraction, we would be remiss if the subject of endotoxins was not again introduced. Readers may recall that all tests conducted on the CDB to date indicate that they are Gram-negative. A Gram-negative test is important for bacteria as it indicates at least three characteristics of importance:
1. The cell walls are lipid-rich in comparison to Gram-positive bacteria.
2. The negative test indicates the presence of lipopolysaccharides (LPS) within the cell wall; lipopolysaccharides are essentially synonymous with endotoxins.
3. Pathogenic bacteria are often associated with endotoxins.
Let us visually compare the cell walls of a Gram-positive bacteria vs. a Gram-negative bacteria:
Source : microbewiki.kenyon.edu
There are distinctive differences that can be noticed. Starting from the bottom, we can see that both cells contain phospholipids (the lipid bi-layer presented earlier). The Gram-negative cell, however, is lipid rich, while the Gram-positive cells have a much lower lipid content. The lipid content of the Gram-negative cell wall is approximately 20-30%, which is very high compared to the Gram-negative cell wall. The relatively high volume of lipids that have been extracted from the CDB are supportive of the Gram-negative test result.
In the Gram-negative cell, the peptidoglycan layer is about 5-20% by dry weight of the cell wall; in the Gram-positive cell the peptidoglycan layer is about 50-90% of the cell wall by dry weight. Peptidoglycan, also known as murein, is a polymer consisting of amino acids and sugars.
Gram negative bacteria are generally more resistant to antibiotics than Gram-negative bacteria. In consideration of the cross-domain terminology currently in use, it is of interest to note that the archaea can be either Gram-negative or Gram-positive; the archaea and the eukaryotes remain under equal consideration within the studies. It is also of interest to know that until relatively recent times that the archaea were classified as bacteria and that the classification systems of biology remain dynamic.
A central difference between the two forms, beyond the relative lipid content and peptidoglycan layer, is the presence of lipopolysaccarides (LPS) on the Gram-negative bacteria. LPS, or endotoxins, elicit a strong immune response in animals.
Aerosolized endotoxins are known to have a significant effect upon the pulmonary system and chronic exposures are known to increase the risk of chronic obstructive pulmonary disease (COPD). COPD is now the third leading cause of death in the United States. Sub-lethal doses cause fluctuations in body temperature (short term increases and longer term decreases), and changes in the blood, immune, endocrine systems and metabolism. They can result in “flu-like” symptoms, cough, headache and respiratory distress. They are linked to increases in asthma and chronic bronchitis. There are no regulatory standards for the levels of endotoxins in the environment (source : National Resources Defense Council).
Endotoxins are associated with increased weight gain, obesity, gum and dental infections and diabetes. A linkage with Chronic Fatigue Syndrome exists, as well as with atherosclerosis, oxidative stress, chronic conditions, cardiovascular disease and Parkinson’s Disease. The condition of endotoxins within the blood is referred to as endotoxemia.
There may be a discomforting familiarity with the above symptoms in correlation with the so-called “Morgellons” condition; this familiarity justifies intensive research into the potential linkage between “Morgellons” and endotoxins.
Lastly, let us now review an infrared investigation into the nature of the extracted lipids.
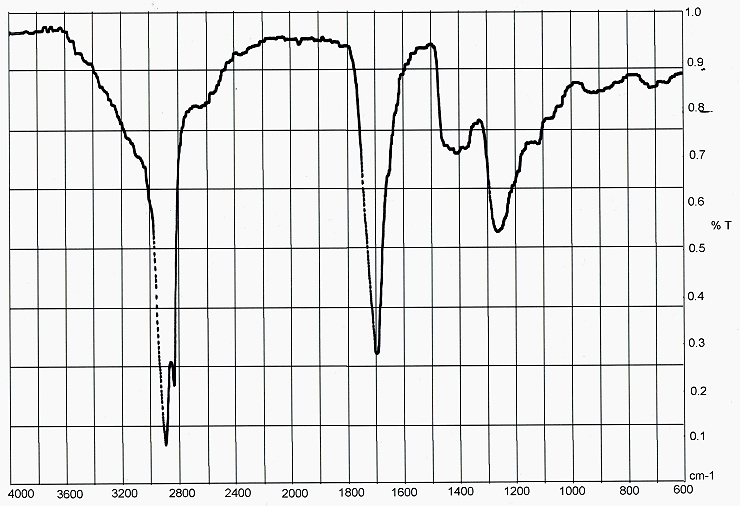
Infrared Spectrum of CDB Lipids
Although a low resolution IR spectrophotometer has been used for this project, a very clear spectrum has been obtained. The spectrum is dominated by peaks at 2900 cm-1 and 1700 cm-1. The 2900 cm-1 peak can be attributed to sp3 single carbon-hydrogen bonds. This functional group is perfectly in accord with the structure that forms the core of a fatty acid, as:
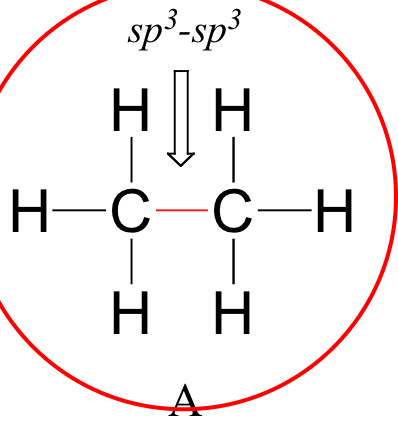
source :http:chemwiki.ucdavis.edu
In addition, the peak at 1700 cm-1 can be attributed to carbon-oxygen double bonding, also in perfect accord with an unsaturated fatty acid, subject to oxidations as extensively described in this report.
A probability model has been developed for the analysis of infrared spectrums, subject to the constraints of the technology available to the Institute. The application of the model to the infrared spectrum above presents the following relative probabilities for the existence of the various functional groups:
| Functional Group | Relative Probability of Existence | |
| Ketones | 90% | |
| Alkanes | 70% | |
| Aldehyde | 60% | |
| Carboxylic Acid | 45% | |
| Phosphonate | 45% | |
| Silane | 37% | |
| Phosphonic Acid | 30% | |
| Ether | 30% | |
| Ester | 30% | |
| Amide | 20% | |
| Phosphine | 20% | |
| Sulfate | 15% |
An analysis of the above probability table will demonstrate that it is highly dominated by the combination and presence of carbon-carbon and carbon-oxygen single and double bonds functional groups. The study and examination of the high probability functional groups and their potential impacts upon health will continue; the strong appearance of the ketone and aldehyde groups with a double carbon-oxygen bond (carbonyl group) is also of high interest here; the aldehydes are very easily subject to oxidation. The potential presence of impurities within the sample will also need to be examined further, including those that might be a part of the extraction process.
All assessments in this report are highly corroborative of one another and they support the assessment of a highly unsaturated lipid, and all that this entails, as comprising a core structure of the CDB extraction that has taken place.
Additional Note:
Some additional analysis of biomolecules with the use of more capable and advanced infrared spectroscopy instrumentation has been completed as of May 2016. The structural information identified continues to support the hypothesis that the CDB derive from the bacterial domain and this remains a primary focal point of research as to its origin. The degree of overlap of genetics, if any, with the remaining archaea or eukaryote domains remains an open topic of research.
Clifford E Carnicom
Mar 12 2015
Edited May 29 2016
born Clifford Bruce Stewart
Jan 19 1953