DRASTIC pH CHANGES
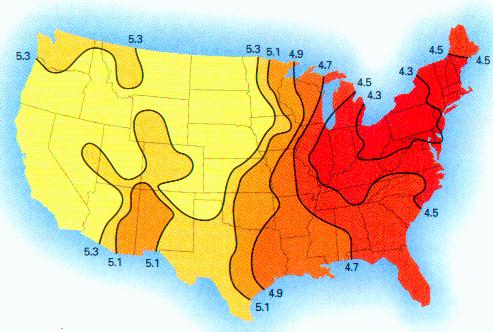
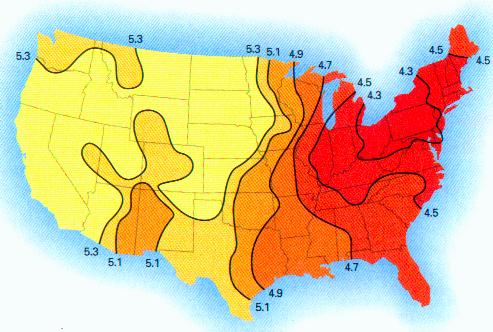
This paper discusses the statistical significance of the measured startling changes in the hydrogen ion concentration in the clouds, precipitation, rainfall in the years from 1990-2000 in the United States. These atmospheric changes are correlated directly with the presence of sustained and extensive aircraft aerosol operations since the beginning of 1999.