The Breaking of Bonds and the Reduction of Iron
Clifford E Carnicom
Nov 03 2012
Note: I am not offering any medical advice or diagnosis with the presentation of this information. I am acting solely as an independent researcher providing the results of extended observation and analysis of unusual biological conditions that are evident. Each individual must work with their own health professional to establish any appropriate course of action and any health related comments in this paper are solely for informational purposes and they are from my own perspective.
Three methods that appear to interfere with the molecular bonding of the iron-dipeptide complex that is now understood to be characteristic of the “Morgellons” growth structure have been established and identified. The iron-protein complex is believed to be of, or similar to, the “Rieske Protein” (iron-sulfur) form. These three methods also appear to be variably successful in reducing the oxidation state of the encapsulated iron from the Fe(III) state to the Fe(II) state. The discovered methods involve the use of ascorbic acid (Vitamin C), N-acetyl cysteine (NAC) and glutathione. The results of applying glutathione appear to be especially promising at this time, as it appears that a major disruption in the bond structure has taken place after approximately 72 hours. The methods have been established and verified through visual, chemical and spectroscopic methods and each has an effect independent of the others. The hypothesis to be made here is that the growth of the organism itself may be interfered with as a result of this work.
This result has been a primary target of research through this past year, and it may represent an important potential inhibitor to the structure growth. The results may also indicate a certain level of coincidence of research or result that has been achieved; an interest in the use of NAC as a mitigating influence (i.e., bio-film reduction) by direct experience has been expressed by independent parties over time1. Synergetic use of all three compounds is also a prospect for investigation. Additional research will seek out the particular chemistry of the disassociation and reduction process (see Additional Note below). Intensive and exhaustive study of the molecular structure of the proteinaceous compound with more sophisticated equipment remains in need.
It will be found that there are important interactions and relationships in the body between cysteine compounds, NAC and glutathione. The combination of influences and interactions between iron, cysteine, histidine, ascorbic acid, N-acetyl cysteine and glutathione represents an important pathway of research for the Morgellons condition. These considerations are to be added to those that have also been outlined in previous research papers on this site in recent years. Full consideration of all information that has been made available may be beneficial in developing any strategies of mitigation for the condition.
All tests have been conducted within a laboratory setting and they do not involve the human body in any fashion; please note the caution at the introduction of this paper. Formidable difficulties remain with the consideration of the highly impervious casing (likely keratin based) that encapsulates the internal growth forms and the environmental forms. The work herein, in combination with the importance of iron within the structure, as well as specific amino acids (cysteine and histidine) that have been identified as a part of the growth form, may represent important milestones in the prolonged research of the so-called “Morgellons” condition.
Listen to a Research Discussion on This Topic
The iron in an oxidized Rieske protein form exists in the Fe(III) state. In the reduced Rieske protein form, one iron atom is in the Fe(III) state and the other iron atom is in the Fe(II) state. The iron-sulfur centers perform a function of electron transfer by alternating between the oxidized and reduced state; this is known as a redox couple. Ascorbic acid and NAC are both known to be powerful reducing agents. Glutathione is also a powerful reducing agent and it is a tripeptide that is known specifically to be able to break disulfide bonds. Disulfide bonds have long been though by this researcher to be a key element of the structural framework of growth. It can be shown directly by chemical tests that ascorbic acid, NAC and glutathione reduce iron from the Fe(III) state to the Fe(II) state. It is anticipated that this fact plays an important role in the protein (dipeptide) bond disruption that has been identified in this report.
The inability of glutathione to be effectively absorbed in the human digestive system is another important consideration; attention must be given to the “precursors” of formation of glutathione, such as N-Acetyl Cysteine ( NAC), denatured whey and cysteine-rich foods. Please refer to the research discussion and the informational videos linked into this report for additional information on this important aspect of this research.
Listen to a Research Discussion on This Topic
Dr. Oz
Glutathione – Master Antioxidant
(3 min)
Dr. Mark Hyman
Glutathione – The Mother of all Antioxidants
(10 min)
David Perlmutter
Glutathione – The Master Antioxidant
Webinar Presentation (1 hr)
(Note the references to N-Acetyl Cysteine (NAC) and whey(lactoferrin) in the second video of the series(Dr. Mark Hyman))
(No endorsements of products to be implied or stated herein)
Dr. David Perlmutter
Glutathion Therapy – Part I (2 min)
Dr. David Perlmutter
Glutathion Therapy – Part II (5 min)
A Demonstration of the Reduction of the Ferric (III) Iron Ion to the Ferric (II) Iron Ion Using Glutathione.
Both test tubes contain a solution of ferric (III) chloride. In addition, glutathione has been added to the test tube on the right. Both test tubes are subsequently tested for the presence of the ferrous(ii) ion. The test tube on the right with glutathione passes this test; the test tube on the left fails the test. This demonstrates the ability of glutathione to reduce iron from the ferric (III) to the ferrous (II) state. The spectrophotometer graphs that follow within this report demonstrate this same phenomenon using developed cultures from oral filaments, i.e, a reduction in the iron oxidation state indicated by a shift of the wavelengths toward the red portion of the spectrum with the addition of glutathione and other anti-oxidants to the culture extracts.
Source : Chemistry for the Life Sciences, Rutton, 2003.
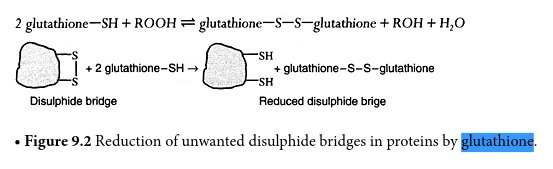
The diagram above shows how gluathione is effective in breaking down disulfide bonds that are an integral part of the growth structure of the cultured forms. Sulfur is indicated by ‘S” in the sketch and the sulfhydryl group (sulfer attached to hydrogen) is indicated by “SH”. The disulfide bond is indicated by the two S units linked together in the left segment of the diagram. The irregular structure is essentially arbitrary in form and could represent a proteinaceous structure, for example (as in this report).
The objective of the graphs below is to demonstrate, in much greater detail, a particular shift in color that takes place with the addition of certain antioxidants (vitamin C, NAC and glutathione). The shift in color occurs (towards the red end of the spectrum, peaking at approximately 490 nanometers (nm)) with the addition of a specific chemical reagent that turns red in the presence of ionic iron (Fe+2 and Fe+3). This test is useful from two standpoints: It detects both the breakdown of the iron-sulfur bonds within the core of the dipeptide and it demonstrates the reduction of the coordinated iron complex to ionic form(Fe+2).
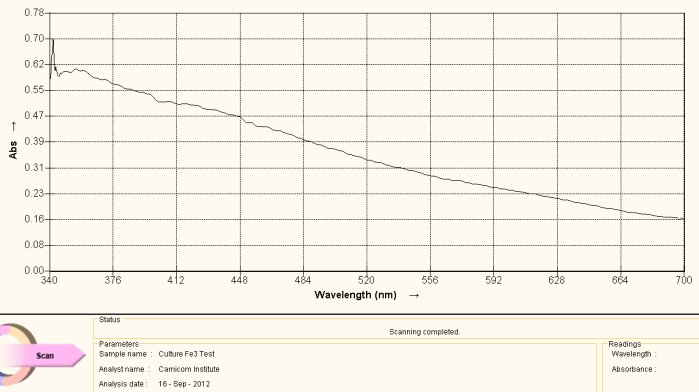
Visible Light Spectrum of Oral Filament Culture Fe(III) Test (negative result)
The graph above serves as a reference spectrum for us. The sample for the spectrum is an extract from the numerous identical cultures that have been developed from oral filament samples.
The extract is formed in the following fashion: The culture is fully developed into the filamentous form. The culture is then removed from the growth medium (in this case red wine), thoroughly rinsed and then dried by subjecting it to low heat for a prolong period. The dried culture is then pulverized to a powder state with mortar and pestle. After drying and pulverization, the sample subjected to a sodium hydroxide (lye) solution along with heating to the boiling point. This process will break down the growth form sufficiently to at least partially dissolve the powdered form to produce a colored solution, which is required for visible light spectral analysis. The heat and lye process is a known method that will break down, at least in part in this case, proteinaceous materials. The color of solution that results is dark brown. This alkaline solution is then filtered into a storage container and exists as a concentrated extract of the original culture.
All information at this time indicates that it can be stored essentially indefinitely in this state without biological degradation; subsequent cultures can be developed further at any time in ease. This result strongly suggests that a “spore-like” state of existence is in place under these circumstances. This fact has actually been demonstrated earlier on many occasions, such as the development of cultures from environmental filaments (e.g, the “EPA” filament) ten years after the original collection. As has been repeatedly expressed, there appears to be no significant chemical , biological or morphological difference between the environmental filament samples the oral filament samples, the skin lesion filament samples and the culture filament samples. This statement alone is profound with respect to expected distribution of the “Morgellons” condition.
The extract that has been created is too concentrated to use directly in spectral analysis; it must be diluted to allow sufficient light to pass through the solution. In the case above, approximately four drops of the extract are placed into approximately three milliliters (ml) of distilled water; the original solution and culture spectrum under the lye-heated treated condition is a pale brown. It is of passing interest that this color alone corresponds well with that of the hydrated ferric ion complex. The subject of iron within the growth form has been discussed at great length in prior reports (e.g., see Morgellons : A Thesis).
For the purpose of this study, the sample is then subjected to a chemical reagent (potassium thiocyanate) that is extremely sensitive to the presence of the ferric iron state, i.e., ionic iron iron in the +3 oxidation state (III). This reagent will cause a solution to turn red in the presence of the ferric ion. Spectral analysis is useful here as it can graphically demonstrate shifts in frequency that may not be apparent to the human eye. In the case above, there is no discernible difference in color by eye or by spectral analysis with the addition of the Fe(III) detection reagent. This proves to us that iron in the ionic state of +3 does not exist in the original culture extract. It says nothing about the presence of iron forms that may exist in other states (e.g. such as a coordinated iron complex) as we shall soon see. These complexities of the iron presence issue have also been discussed at length in the previously mentioned report.
The spectrum is then taken after the Fe(III) reagent detection addition and the result is shown above as a reference spectrum. What we observe in the visible light portion of the spectrum is a fairly monotonic and steady decrease in absorbance as the wavelength decreases. It would be of great value to extend this spectral analysis into the infrared and ultraviolet regions, but this equipment is considerably more expensive to obtain and it is not yet available to the Institute. The resources to expand the current analysis remain in great need. This spectrum, nevertheless, is of great value as a reference point, and it suffices to be able to detect the shifts in frequency that result from the anti-oxidant studies that are the basis of this report.
Visible Light Spectrum of Oral Filament Culture Fe(II) Test (negative result)
The description for the graph above is identical to that of the previous one with one important exception. In this case the reagent that has been applied (1,10 phenanthroline) is a test for the presence of the ferrous (Fe+2) iron state(II) within the culture extract vs. the ferric (III) form. This test result is also negative and it likewise shows that iron (III) does not exist in ionic form within the culture extract. Equally, it says nothing about the existence of iron in another form (e.g. ionic or complexed state) within the culture extract. The importance of the state of iron that exists within any compound or complex is therefore paramount and it will be demonstrated further later in this report.
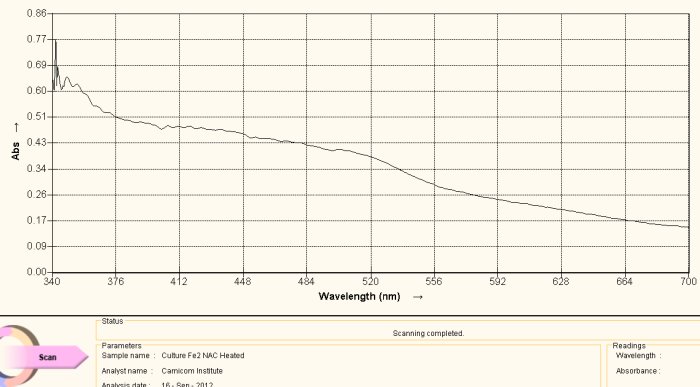
Visible Light Spectrum of Oral Filament Culture Fe(II) Test NAC (positive result)
Note shift toward the red portion of the spectrum.
This spectrum shows an important difference with the two reference graphs that have been previously presented. This particular sample has N-acetyl cysteine (NAC) applied to the culture extract in addition to the reagent for the detection of iron (III). What is important to observe here is s the shift in frequency toward the red portion of the spectrum. What this means, in simpler terms, is that iron in the ferrous ionic state (III) has now been detected.
This change means that two very important changes have taken place. First, the bonds that hold the iron within the growth structure have been broken to release the iron in the first place. Secondly the iron is the the reduced state of Fe(+2), or in the state that is again able to combine with oxygen in hemoglobin. These two findings potentially represent distinct advantages in the quest to “interfere” with the growth stages of this (i.e., “Morgellons” associated) organism. Thirdly, this particular anti-oxidant (reductant) can be incorporated into general health improvement regimens as it is already widely known and used to that end in the health and medical communities.
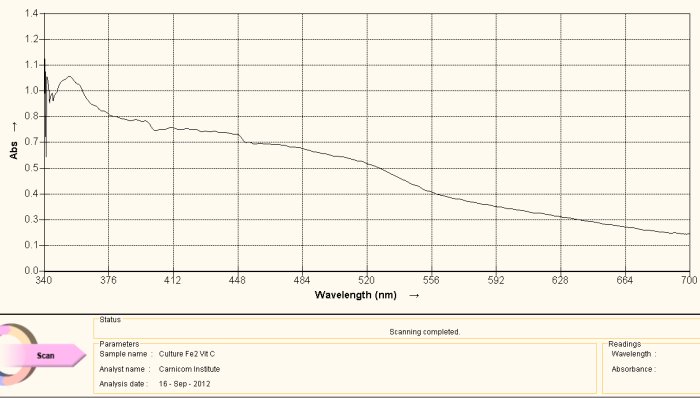
Visible Light Spectrum of Oral Filament Culture Fe(II) Test Ascorbic Acid (positive result)
Note shift toward the red portion of the spectrum.
The discussion as it relates to NAC above can be applied equally in this case, but again with one important exception. In this case, the added agent to the culture extract is ascorbic acid, or Vitamin C. All conclusions above made with respect to the use of NAC above remain similar and valid with the use of ascorbic acid. The reduction level in both cases appears to be relatively weak, i.e. the shift in color is also detectible by eye, but barely so. The ability to break the iron-disulfide bond complex (as concluded by the detection of iron oxide and cysteine within the complex) and to reduce the iron to a free +2 state is of potentially monumental significance.
It is also wise to recall earlier research that indicated a level of effectiveness in inhibiting the growth of the culture using ascorbic acid as well. That earlier research may be deserving of additional attention and repetition in light of these current findings. It may well be found that there will be associated relationships, particularly in the anti-oxidant (i.e., reductive) qualities of ascorbic acid. Recollection of the mechanisms involved in the “Fenton” reaction may also serve us well in the near future and they too have been described within earlier research.
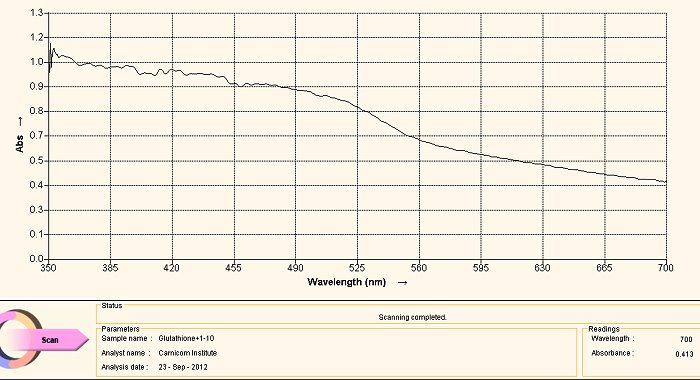
Visible Light Spectrum of Oral Filament Culture Fe(II) Test Glutathione (positive result)
Note shift toward the red portion of the spectrum.
The case of using glutathione as an anti-oxidant (or as a reducing agent) is an especially interesting one. The call to consider the use of glutathione was based upon research related to the need to break down disulfide bonds, and as such it arose from a strongly directed proposition. The results have been favorably surprising and they have led to an in-depth interest in glutathione and to how it affects our health in the main.
With respect to the spectrum immediately above, we can actually regard it as being essentially equivalent to the two previous cases, i.e., NAC and ascorbic acid. There is indeed a shift in the wavelength towards the red end of the spectrum (indicating the presence of Fe(II), but it continues to affect the culture in a relatively weak sense. Our pleasant surprise comes with the final spectrum shown below.
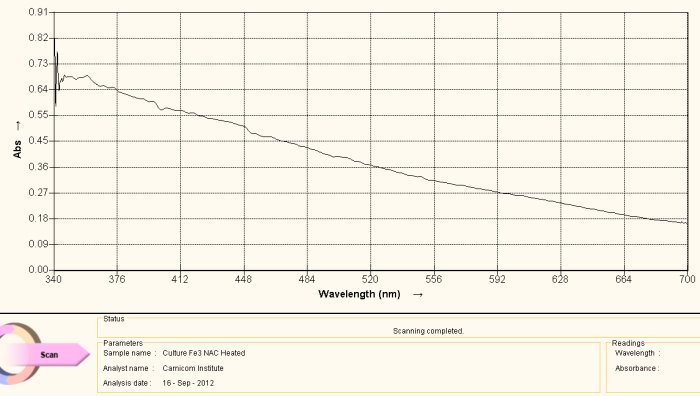
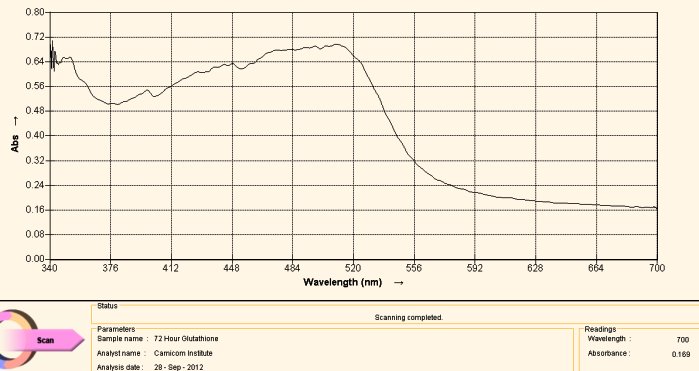
Visible Light Spectrum of Oral Filament Culture Fe(II) Test Glutathione after 72 hours. (positive result)
Note major shift toward the red portion of the spectrum.
This last case, again involving the use of gluthathione as a reducing agent, has come about by “accident”. Many acts of discovery seem to follow that fortuitous path. What has happened here is that the test tube was allowed to sit for approximately three days. At the end of this period the extract solution had turned a bright and visible red. The spectrum above is another way of verifying this same fact; peak absorbance has been clearly, strongly and definitively shifted toward the reddish portion of the spectrum, peaking at approximately 500 nm. The reddish color appears, again, because of the induced presence of the ferrous iron ion by the glutathione, in addition to the breaking of the bonds that encapsulate (or chelate) this same iron.
What this means, also in simpler terms, is that glutathione (with the sufficient passage of time) is quite effective in breaking down the bonds in the proteinaceous complex that has been identified within the “Morgellons” growth form. In addition, it appears equally and concomitantly effective in releasing and reducing the free iron to a +2 oxidation state. Iron is required to be in the +2 oxidation state to bind to oxygen within hemoglobin.
The aggregate impact of these anti-oxidants (reducing agents) does hold some promise for us. Interference with the bond structure of the dipeptide that has been identified, along with the release of the chelated iron within the complex, have been the primary goals of this researcher for some time now. The continued pursuit of this strategy appears to remain as a wise choice of energy, effort and research. The pace and depth of this research can be increased with proper support, should it ever come to pass. There remain other viable strategies as well to be explored.
Readers , as always, are advised to consult with their health practitioners as to how this information may be best put to use. For instance, the complexities of glutathione actions within the body do not allow for simple “replacement by supplement”. There is a host of knowledge about the “precursors” of glutathione that is required to more effectively use the information and discoveries within this report. Nevertheless, the role of anti-oxidants (reducing agents) with particular emphasis upon glutathione, N-acety cysteine and ascorbic acid appears to be justified with promise at this stage. Readers may wish to avail themselves of additional mitigating strategies that have been enumerated within the numerous research papers on the Morgellons issue within this site.
Sincerely,
Clifford E Carnicom
October 27, 20122,3,4
Additional notes:
1 Appreciation is extended to Sandra Autry, former Carnicom Institute Associate Member, for her interests, recommendations and experiences expressed on this topic – Personal conversation.
2. Many thanks to Carol Carnicom for her recent expressive cello playing in Devorak’s New World Symphony, which served as a dramatic and energetic backdrop to one of the sessions for this report.
3. This paper authored by Clifford E Carnicom, originally entering this world as Clifford Bruce Stewart, born January 19, 1953. Call it a journey of adoption.
4.Many thanks to the Pecos River at Villa Nueva in New Mexico, where the vestiges of Indian summer have graciously closed down this report..