MORGELLONS :
GROWTH INHIBITION CONFIRMED
Clifford E Carnicom
Mar 15 2010
Note: I am not offering any medical advice or diagnosis with the presentation of this information. I am acting solely as an independent researcher providing the results of extended observation and analysis of unusual biological conditions that are evident. Each individual must work with their own health professional to establish any appropriate course of action and any health related comments in this paper are solely for informational purposes and they are from my own perspective.
The growth of the bacterial-like organisms that appear to be at the foundation of the so-called Morgellons condition has been positively inhibited. The basis of the rationale that is used in these trials has been outlined in detail in a previous report entitled Morgellons : A Discovery and a Proposal1. The basis of that report is the application of a set of specific antioxidants that inhibit the growth of the organism(s) in the presence of the hydroxyl free radical and the creation of a more alkaline environment. It has been established in that earlier report that the organism(s) thrive in an acidic environment in the presence of the hydroxyl radical and oxidizers in general.
The basic strategy that has been adopted is a transformation of the growth environment to a more alkaline condition along with adding specific antioxidants that are directed toward the scavenging of the hydroxyl radical. Please also refer to the earlier paper for the rationale behind the selection of the particular antioxidants that have been used.
There is absolutely no statement herein that indicates the particular organism(s) has been terminated or extinguished, only that growth of the organism(s) under the specific conditions and trials mentioned has been inhibited. There is no assurance that all agents used in these trials is required to produce these results, nor that they be used at the arbitrary dosage levels that have been chosen for the cultures. Future work will examine the reduction or restriction of these same agents and dosages with the goal of replicating the results.
This paper shall be brief as it confirms the proposal of the preceding paper more explicitly. The primary purpose of the paper will be to demonstrate the inhibition that takes place in confirmation of the earlier work and to enumerate the specific antioxidants that have been used in these trials. There remains an overwhelming amount of work that remains to be done, and these results simply promote one particular strategy that is worthy of exhaustive and intense study. It is anticipated that other antioxidants that emphasize scavenging the hydroxyl radical and that alkalize the growth environment may also be effective.
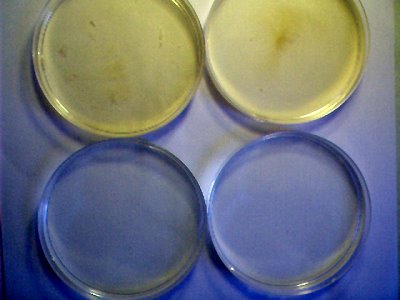
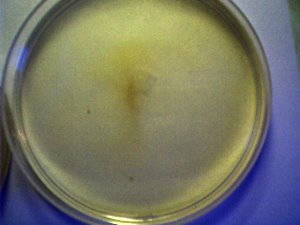
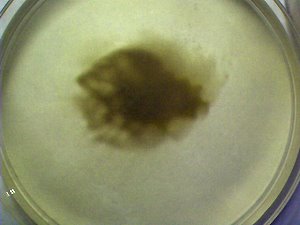
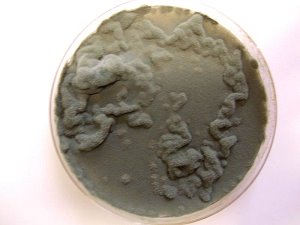
PHOTOGRAPHS
|
A more advanced stage of the bacterial-like (chlaymidia-like and mycoplasma-like) growth of the culture under conditions identical to that immediately above. This culture is approximately 1-2 weeks old and is in white wine. The success and advantages of the white wine and clear culture (simulated wine) has been previously described. |
The more advanced stage of surface filament growth in a wine culture medium as has been reported on extensively and as developed by an independent researcher that is in the process of duplicating a portion of this work. This photograph represents the first presentation of the filament stage of growth in a white wine vs. a red wine environment. This demonstrates the lack of dependence upon the color of a red or white wine to produce this culminating stage of growth. This culture has been developed from a separate red-wine filament culture and not from the bacterial stage exhibited above. This filament growth is identical to that which originates from the dental sample cultures that have been reported on extensively in this site. The filament growth exhibited here has also been shown to be identical in form, size and structure to that developed from certain environmental samples, namely that which has been refused for identification by the U.S. Environmental Protection Agency. |
Additional notes:
Note: I am not offering any medical advice or diagnosis with the presentation of this information. I am acting solely as an independent researcher providing the results of extended observation and analysis of unusual biological conditions that are evident. Each individual must work with their own health professional to establish any appropriate course of action and any health related comments in this paper are solely for informational purposes and they are from my own perspective.
The white wine medium in each dish is 30 ml. At this point, no distinctions in growth have been determined between different varieties of wine, either red or white. The white wine cultures offer the advantage of clarity in observation.
Some reports on toxicity levels of ascorbic acid and Vitamin C reported on are as follows:
| “Since ascorbic acid is a water-soluble vitamin, toxic levels are not built up or stored in the body, and any excess is lost mostly through urine. If extremely large amounts are taken gastrointestinal problems may appear, but will normalize when the intake is cut or reduced. To determine a level where a person might experience discomfort is difficult, since some people can easily stomach up to 25,000 mg per day, while others start having a problem at 600 or 1,000 mg.”3 |
| “Vitamin C exhibits remarkably low toxicity. The LD50 (the dose that will kill 50% of a population) in rats is generally accepted to be 11.9 grams per kilogram of body weight when taken orally.[56] The LD50 in humans remains unknown, owing to medical ethics that preclude experiments that would put patients at risk of harm. However, as with all substances tested in this way, the LD50 is taken as a guide to its toxicity in humans and no data to contradict this has been found.”4 |
Approximately 30 mg. of ascorbic acid has been added to the volume of 30 ml of white wine (approx. 1000 mg. / kg of solution). Equating this roughly to the human body (assume 70 kg.), this translates to a single dosage of approximately 70 gms. Assuming an ingestion of 1000 mg per day, this equates to distributing the above dosage over a period of approximately 70 days to reach the equivalent result. An ingestion rate of 10,000 mg. of ascorbic acid per day leads to a time period of approximately 7 days to reach an equivalent result.
This example points out the outstanding and continuous need for all individuals to consult with their own medical professionals to manage their own individual health requirements and objectives; I have not and I will not provide any medical or diagnostic advice. I have reported and I will report on laboratory conditions and the results achieved from that work.
Approximately 0.1 ml (~.126gms.) of glycerol (USP) (glycerine) has been added to the volume of 30 ml. of white wine (equates to approx. 4.2 gms / kg.).
With respect to glycerol, some of the toxicity information available is as follows:5
| ” IPR-RAT LD50 8700 mg kg-1 ORL-RAT LD50 12600 mg kg-1 SCU-RAT LD50 100 mg kg-1 ORL-MUS LD50 8700 mg kg-1:” |
Additionally,
| “A recent GLP compliant oral gavage study in rats given glycerol formal for 90 days at dosages up to 25 mg/kg indicated no treatment changes in physical signs of animals, bodyweight gain, hematological, biochemical or urine analysis.”6 |
To equate 25 mg. / kg. as referenced in the latter report to a human body, this equates to a daily intake of approximately 1.75 gms. / 70 kg.
From the former report, LD50 (lethal dose 50% probability) orally of glycerol is therefore approximately 12.6 gms / kg. for rats. This equates to approximately 882 gms. per 70 kg. of the human body. At 25 mg. / kg., 4.2 gms. / kg. is to be distributed over a period of approximately 168 days to reach an equivalent dosage.
Approximately 0.25 ml of sodium citrate solution has been added to the volume of 30 ml. of white wine. The sodium citrate solution has been prepared by combining lemon juice with baking soda to reaction completion.
With respect to the toxicity of sodium citrate, the following is identified:
| “LD50: Oral rat LD50 >8 g/Kg“7 |
This equates to the human body in mass at approximately > 560 gms / 70 kg. Sodium citrate is an alkalizing agent, may have interactions with other ingredients or compounds and its potential application must be coordinated and directed though medical consultation8,9. If any information in this section is found to be incorrect or requires revision, please contact me at [cec102@usa.com] with the appropriate and supporting documentation. Future trials will consider reductions in dosage since at this point the dosage reference levels are entirely arbitrary. This paper terminates with the commencing condition of release:
Note: I am not offering any medical advice or diagnosis with the presentation of this information. I am acting solely as an independent researcher providing the results of extended observation and analysis of unusual biological conditions that are evident. Each individual must work with their own health professional to establish any appropriate course of action and any health related comments in this paper are solely for informational purposes and they are from my own perspective.
References:
1. Carnicom, Clifford, Morgellons : A Discovery and a Proposal, http://carnicominstitute.org/wp/7471-2/, Feb 22, 2010.
2. Carnicom, Feb 22.
3.Ascorbic Acid – Vitamin C – Information, http://www.anyvitamins.com/vitamin-c-ascorbicacid-info.htm
4. Vitamin C, Wikipedia, http://en.wikipedia.org/wiki/Vitamin_C
5.Safety Data for Glycerol, http://msds.chem.ox.ac.uk/GL/glycerol.html
6.Committee for Veterinary Medicinal Products, Glycerol Formal Summary Report, http://www.ema.europa.eu/pdfs/vet/mrls/010896en.pdf
7.MSDS, Aqua Science Inc., http://aquascience.thomasnet.com/Asset/31-244_FerroVer.pdf
8. Citric acid and sodium citrate, http://health.yahoo.com/urinary-medications/citric-acid-and-sodium-citrate/healthwise–d03952a1.html
9. Citric acid-Sodium citrate, http://www.healthline.com/goldcontent/citric-acid-sodium-citrate