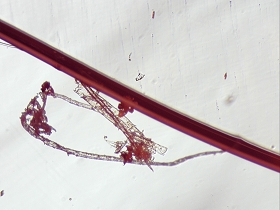
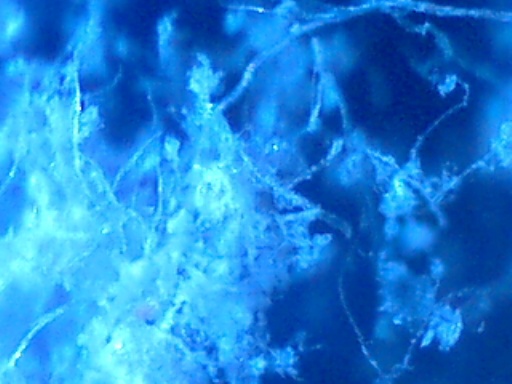
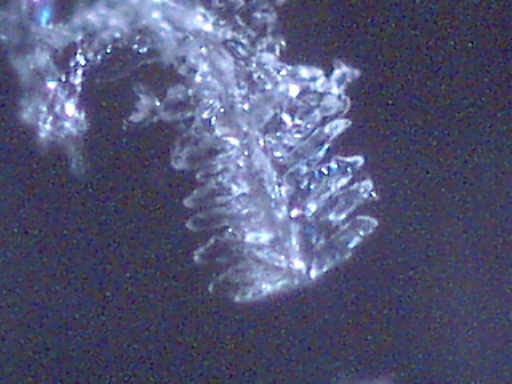
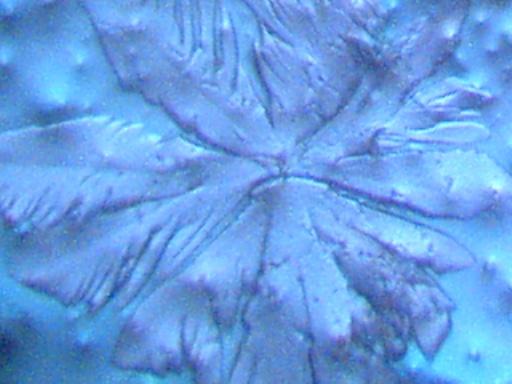
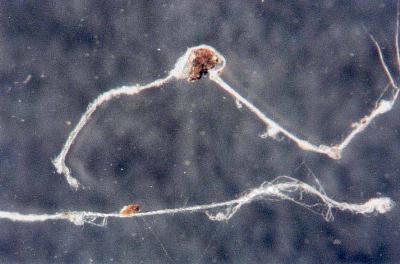
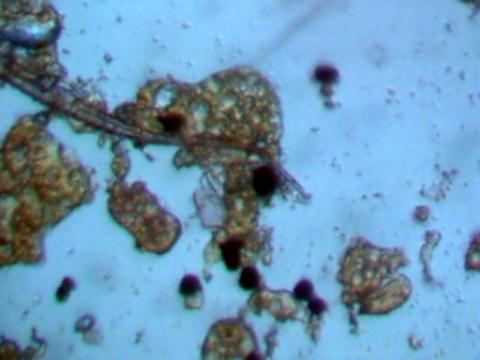
Crystalline structures that were found aligned on a driveway in Carpinteria, California on June 17, 2003 are examined in this work. A concerned citizen contacted Clifford Carnicom, who recommended the samples be sent to Carnicom for analysis. These structures are rectangular/cubicle, opaque, translucent, and insoluble in water, hydrochloric acid, sulfuric acid, sodium hydroxide and acetone. Pictures of the as now unidentified material are included for analysis.