This paper is Part Two of a Six Part series.
A series of means and methods have been developed to examine the state of human blood samples. This additional work was completed between February and May of 2022 in a field setting with portable instrumentation. The work was motivated by the rather profound blood coagulation activity recorded in a previous paper, Blood Alterations I : Coagulation (Jul 2022). The record of observations for this work is contained in Carnicom Institute Laboratory Notebooks, Vol 26 and 27; these volumes have been made available to the public through this site. The work is extensive and detailed in nature and the laboratory notes will serve as the most accurate record of what transpired; this and forthcoming research papers will be of a summary nature.
The entire set of laboratory notebooks is also available within this site.
Future papers will discuss the nature of any changes that have been observed, and the chemical constitution of such change, as far as is permitted within the means and methods used. Additionally, the implications of such change will be brought forth.
Some of the methods which have been used in these current studies include:
1. Electrochemical analysis
2. Visible light spectrometry
3. Near infrared spectrometry
4. Microscopy
5. Protein detection (reagent based)
6. Enzyme analysis
7. Centrifugation
8. Magnetism
A few comments on some of the above methods and techniques will be helpful in understanding the work that will be discussed in future papers.
1. Electrochemical analysis:
Electrochemistry will be at the heart of the analysis that has emerged here. It is a very powerful tool, especially so in the case of portable and field operation. It is also a very complex and younger discipline with much more to be discovered in terms of application and protocol. The discipline, as in most others in science, is worthy of a profession and career in its own right. One of the great established benefits of electrochemistry is its application to inorganic chemical analysis. The detection of inorganics in chemistry can be a rather exclusive pursuit often requiring very expensive and specialized equipment (e.g., Gas Chromatography – Mass Spectrometry, Inductively Coupled Mass Plasma Spectrometry(ICMP), etc.) Infrared equipment, at least relatively more accessible, is well suited to organic analysis, but not so strongly suited to inorganic analysis. A typical example of inorganic analysis would be the detection and identification of ionic metals, for example. Electrochemistry offers a viable alternative to the needs at hand on some occasions.
At the core of much of electrochemical work will be the examination of oxidation-reduction reactions in chemistry. This is one of the most important fields of study in the physcial, chemical and biological world as it focuses on the transfer of electrons. The transfer of electrons in many ways equates to the transfer of energy in general, and thus its importance in the world around us.
[As an aside, few people may be aware that on one occasion, Carnicom Institute did gain access to an Inductively Coupled Mass Spectrometry laboratory report; this report is actually of great significance. Two years of negotiation in confidence on behalf of the public interest were required to gain this access. The sample, in this case, is the infamous “Environmental Filament” material that has been the subject of intense effort now extending over decades. This is the same material refused for identification by the U.S. Environmental Protection Agency (see EPA Refuses to Identify, Returns Sample 18 Month Delay, Jul 2001).
To review the results of the ICMP analysis, please examine the following paper:
Environmental Filament Project, Metals Testing Laboratory Report, Aug 2017. I would conjecture that few individuals are aware of or understand the special value and significance of this professional laboratory certified report.]
Continuing…
To demonstrate the scope of electrochemistry that is now available, the following is a listing of some of the many methods that have evolved in this science:
1. Linear Sweep Voltammetry
2. Differential Pulse Voltammetry
3. Square Wave Voltammetry
4 .Normal Pulse Voltammetry
5. AC Voltammetry
6. Cyclic Voltammetry
7. Potentiometric Stripping Analysis
8. Amperometry
9. Pulse Amperometry
10. Fast Amperometry
11. Potentiometry / Open Circuit Potentiometry/Chronopotentiometry
12. Multistep Amperometry
13. Multistep Potentiometry
14. Electrochemical Impedance Spectroscopy(EIS)
Cyclic Voltammetry would appear to be the classic application most readily discussed in the literature of textbooks on the subject. Much of my work in the past with this instrumentation has been exploratory, with an emphasis adopted upon AC Voltammetry and EIS. Important achievements were made in past years in the analysis of rainwater samples using AC Voltammetry and also in “Environmental Filament” studies using EIS. Much of my work I would regard, out of necessity, as original in terms of protocol development.
The work described here will center upon the techniques of AC Voltammetry and Chronopotentiometry. A very brief mention of these two divisions of electrochemistry follows.
Chronopotentiometry is a relative simple technique, and it involves subjecting a conductive solution to a varying direct current (DC) voltage over time; the corresponding variable current may also be measured. The instrumentation under use can acquire measurements with high accuracy and sensitivity. Some information about electron transfer within the solution (i.e., oxidation-reduction) may result from such a study. One common goal of any electrochemical study is to better understand the chemistry of that solution by identifying the oxidation-reduction chemical reactions that take place within it. This furthermore can often lead to the identification of specific chemical constituents within that same solution. An example of a what a chronopotentiogram can look like follows:
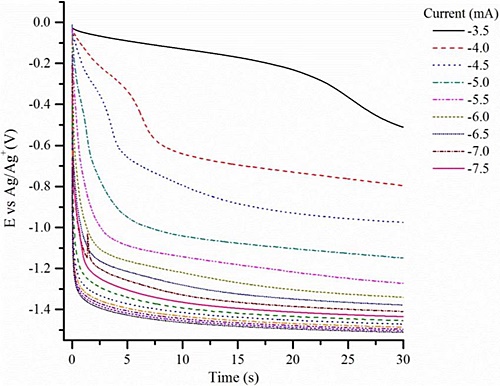
A very standard method of electrochemistry is that of cyclic volammetry; in this case the sample is subjected to a variable DC voltage over time and the current flow is measured. What distinguishes this method is that the polarity of the voltage is then reversed and the measurement process repeated. This therefore can initiate both both oxidation and reduction reactions within the measurement process.
A method used to great extent in this work is that of AC voltammetry. This is a more complex electrochemical method and it requires more sophisticated instrumentation. In this case you have a similar scenario with that of a varying DC voltage over time along with measured current flow. The variation in this method, however, involves the addition of a low magnitude alternating current (AC) signal to the DC signal. This has the effect of dramatically increasing the sensitivity of detection of chemical activity within the solution. This researcher has found it to be a very powerful chemical detection and identification technique over the years, especially involving the study of inorganic compounds in a redox (reduction-oxidation) environment. It is a very powerful chemical analytic technique that can offer additional advantages in cost involved, portability, and field operation. A couple of images below demonstrate the principles of the method along with an example graph of a result:
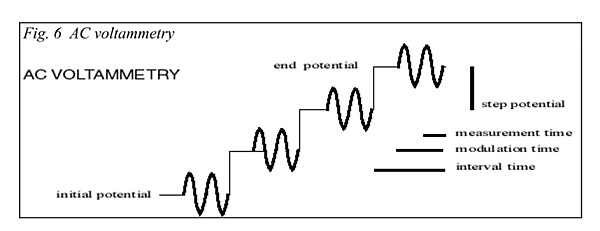
Source: User Manual for Electrochemical Methods – Eco Chemie B.V.
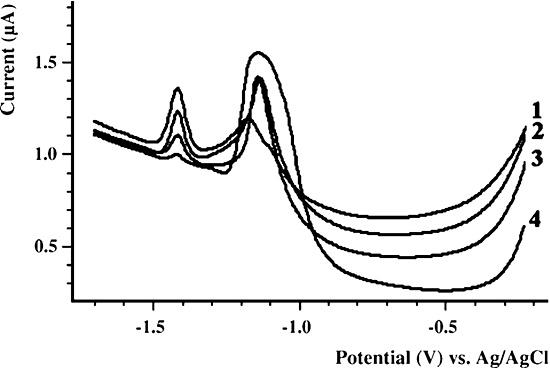
The examination of the peaks and variations in slope on an AC voltammogram are a valuable analytic electrochemical method; they will be of fundamental importance to the information that is to come forth within this report series.
Listings of known reduction-oxidation values of a wide range of chemical reactions are established and published; the listings can vary considerably in their comprehensiveness.
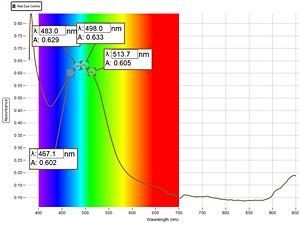
2. Visible light spectrometry:
The portable visual light spectrometer used has a range of 380 – 950 nm, with a resolution of 2 nm. This covers the visible light range, as well as a very minor extension into the near infrared range. Visible light spectrometry is generally of value for solutions of color. While the majority of organic solutions are colorless, blood is a fortunate exception and is therefore a candidate for this type of spectrometry.
3. Near infrared spectrometry:
The portable near infrared spectrometer has a range of 900 -1700 nm, with a resolution of 2 nm. This instrument is sufficient to acquire at least some usable information on functional groups that are likely to exist with a sample. Water in general needs to be removed from the sample to be most effective. The instrument is also useful to establish uniqueness and/or distinction of a sample, among other applications.
4. Microscopy:
The portable microscope used is of modest but important capability (~1500 to 4000x magnification, 1500x generally preferred). Microscopy is an absolute requirement for the fundamental observation of a sample. It is the starting point for most laboratory analysis.
5. Protein detection (reagent based):
One of the essential aspects of the work here is the ability to detect the existence of protein in a sample in a relatively simple fashion, along with variations in chemical constitution or concentration that might be taken place. The development of a suitable chemical color reagent for this need is most valuable. The Bradford reagent is a standard reagent and it has been utilized on many occasions in the past.
Carnicom Institute has, in the past, performed original research on the development of a more sensitive and stable reagent for protein detection. This work was successful in developing a reagent which appears to be at least an order of magnitude more sensitive to protein detection than the Bradford trials were. In one sense, the reagent developed may be viewed as a modification of the Bradford reagent with a maintenance of its core composition. The reagent developed is quite sensitive to determine the existence of a soluble protein. The modified reagent development is recorded within the laboratory notes of the Institute, and the reconstruction of the reagent for this project was fully successful. Furthermore, the combination of a more sensitive protein detection reagent combined with visual light spectrometry becomes a very valuable laboratory technique of protein analysis. This was done to great advantage within the current blood coagulation investigation.
6. Enzyme analysis:
Enzymes are of prime importance in the digestion and breakdown of proteins. They take on special importance in the current investigation and astute readers may already have a sense of that importance which is forthcoming and to be discussed in this article series. Readers will find several references to the importance of enzymes within previous Carnicom Institute Research, including the following papers:
Carnicom Institute Newsletter – Jun 2019
Morgellons : A Supplemental Discussion – Jan 2017
CDB Lipids : An Introductory Analysis – Mar 2015
Morgellons : A Thesis – Oct 2011
Morgellons : A Status Report – Oct 2009
The laboratory method to be referenced here is to mention that changes in protein concentration or composition due to the application of enzymes can be determined with the combined use of enzymes, protein detection reagent, and visual light spectrometry. This laboratory method, employed within this research set, provides a conceptual basis for a future discussion of mitigation strategies.
7. Centrifugation:
Centrifugation is a fundamental laboratory technique useful for the separation of components within a mixture or solution. The separation achieved is a function of varying density of the components.
8. Magnetism:
The issue of magnetism and the application of magnetic forces to various samples is only at an introductory level within this research paper set. Subsequent research, if it occurs, will likely bring this subject to the forefront of inquiry.
Please distribute and preserve this report globally. Thank you.
Clifford E Carnicom
Born Clifford Bruce Stewart, Jan 19 1953
Jul 18 2022